| CPC A61M 1/3679 (2013.01) [A61M 1/0281 (2013.01); A61M 1/14 (2013.01); A61M 1/3687 (2013.01); B01J 20/20 (2013.01); B01J 47/02 (2013.01)] | 20 Claims |
|
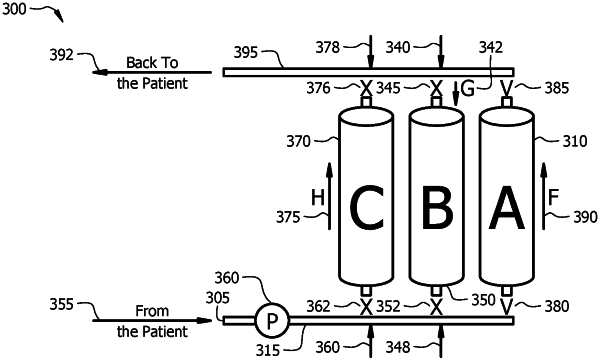
1. A method comprising:
contacting a bodily fluid with an adsorbent material consisting essentially of a synthetic carbon particle (SCP) to produce a first filtrate, wherein the bodily fluid comprises a level of disease mediators (y);
contacting the first filtrate with an adsorbent material consisting essentially of the SCP and an anion exchange resin where the weight ratio of SCP to anion exchange resin is in a range from 0.1:100 to 100:0.1 to produce a second filtrate;
contacting the second filtrate with an adsorbent material consisting essentially of the SCP and a cation exchange resin where the weight ratio of SCP to cation exchange resin is in a range from 0.1:100 to 100:0.1 to produce a third filtrate; and
administering the third filtrate to the subject.
|