| CPC A61L 27/3608 (2013.01) [A61L 27/365 (2013.01); A61L 27/3616 (2013.01); A61L 27/3683 (2013.01); A61L 27/56 (2013.01); A61L 2430/02 (2013.01); A61L 2430/40 (2013.01)] | 20 Claims |
|
1. A method of making an implantable biomaterial for aiding bone regeneration, the method comprising:
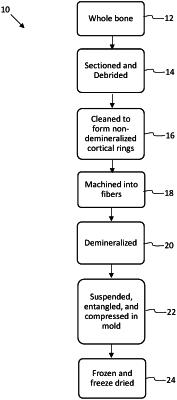
obtaining whole bone;
sectioning and debriding the whole bone into cortical rings;
machining the cortical rings into cortical fibers having lengths of 10-50 mm, widths of 0.5-2 mm, and thicknesses of 0.1-1 mm;
demineralizing the cortical fibers to form demineralized cortical fibers;
suspending and entangling the demineralized cortical fibers in an aqueous solution;
compressing the demineralized cortical fibers in solution in a mold to form a molded biomaterial composition of a given shape;
freezing the molded biomaterial composition; and
freeze-drying the molded biomaterial composition to form the implantable biomaterial;
wherein the cortical rings are cleaned with a 0.05-0.15% v/v sodium deoxycholate solution, a 2-6% hydrogen peroxide solution, and an isopropyl alcohol solution.
|