| CPC A61K 47/549 (2017.08) [A61K 31/713 (2013.01); A61K 47/554 (2017.08); A61K 47/59 (2017.08); A61K 47/60 (2017.08); C07C 235/10 (2013.01); C07C 237/08 (2013.01); C07H 15/08 (2013.01); C07H 15/18 (2013.01); C07H 15/26 (2013.01); C07H 21/00 (2013.01); C07J 51/00 (2013.01); C07J 63/008 (2013.01); C12N 15/113 (2013.01); C07D 207/08 (2013.01); C07D 207/12 (2013.01); C07D 211/42 (2013.01); C12N 15/111 (2013.01); C12N 15/87 (2013.01); C12N 2310/14 (2013.01); C12N 2310/315 (2013.01); C12N 2310/321 (2013.01); C12N 2310/346 (2013.01); C12N 2310/351 (2013.01); C12N 2320/32 (2013.01)] | 11 Claims |
|
1. The compound:
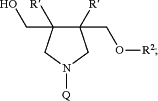 or a salt thereof, wherein Q is -L1-R1;
L1 is a divalent, branched or unbranched, saturated or unsaturated, hydrocarbon chain, having from 1 to 14 carbon atoms, wherein one or more of the carbon atoms in the hydrocarbon chain is optionally replaced with —NRX—C(═O)—, or —C(═O)—NRX—, and wherein RX is hydrogen or (C1-C6)alkyl, and wherein the hydrocarbon chain, is optionally substituted with oxo(═O); or
L1 is selected from the group consisting of:
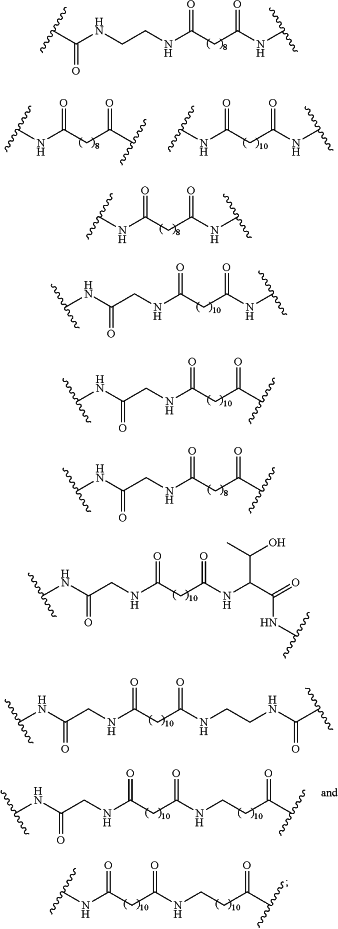 R1 has the following formula:
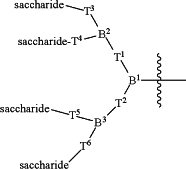 wherein:
B1 is CH;
B2 is selected from the group consisting of:
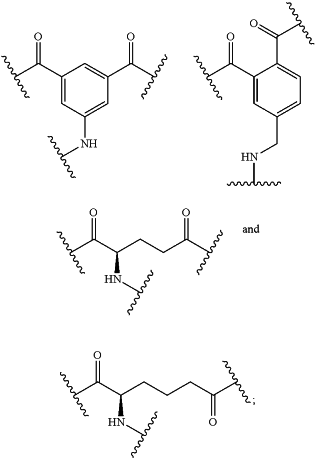 B3 is selected from the group consisting of:
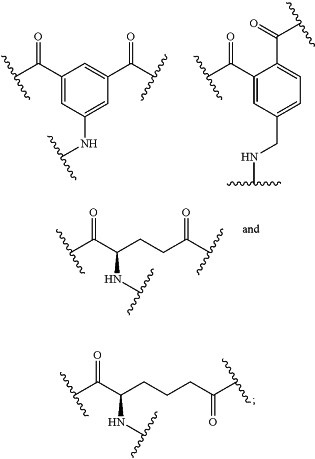 T1 is a branched or unbranched, saturated or unsaturated, hydrocarbon chain, having from 1 to 20 carbon atoms, wherein one or more of the carbon atoms in the hydrocarbon chain is optionally replaced by —NRX—C(═O)—, or —C(═O)—NRX—, and wherein RX is hydrogen or (C1-C6)alkyl, and wherein the hydrocarbon chain, is optionally substituted with oxo (═O), that is covalently bonded to B1;
T2 is a branched or unbranched, saturated or unsaturated, hydrocarbon chain, having from 1 to 20 carbon atoms, wherein one or more of the carbon atoms in the hydrocarbon chain is optionally replaced by —NRX—C(═O)—, or —C(═O)—NRX—, and wherein RX is hydrogen or (C1-C6)alkyl, and wherein the hydrocarbon chain, is optionally substituted with oxo (═O), that is covalently bonded to B1;
each of T3, T4, T5, and T6 is independently selected from the group consisting of:
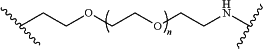 n=1, 2, or 3;
each saccharide is independently selected from the group consisting of:
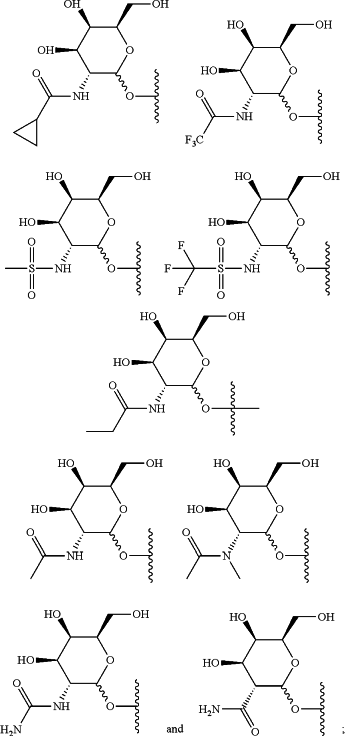 R2 is a siRNA substituent; and
R′ is C1-9 alkyl, C2-9 alkenyl or C2-9 alkynyl; wherein the C1-9 alkyl, C2-9 alkenyl or C2-9 alkynyl are optionally substituted with halo or hydroxyl.
|