| CPC A61B 5/4848 (2013.01) [A61B 5/0035 (2013.01); A61B 5/0036 (2018.08); A61B 5/055 (2013.01); A61B 5/245 (2021.01); A61B 5/369 (2021.01); A61B 5/372 (2021.01); A61B 5/4094 (2013.01); A61B 5/7267 (2013.01); A61B 5/7275 (2013.01); A61B 6/5247 (2013.01); G01R 33/4808 (2013.01); G06N 3/04 (2013.01); G06N 3/08 (2013.01); G16H 10/60 (2018.01); G16H 20/70 (2018.01); G16H 50/20 (2018.01); A61B 6/037 (2013.01); G01R 33/4806 (2013.01)] | 24 Claims |
|
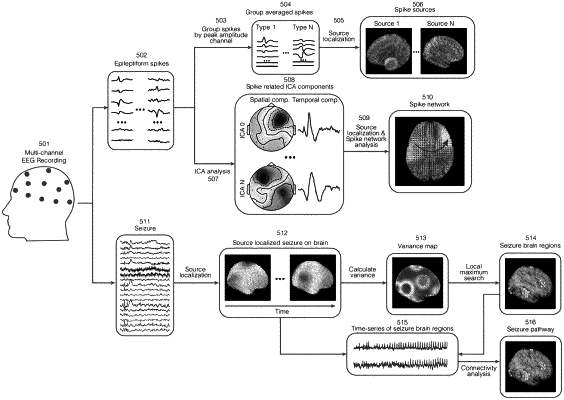
1. A method comprising:
collecting individual patient data from a patient using an electroencephalogram (EEG) system or magnetoencephalogram (MEG) system, wherein the individual patient data includes data of a neural status of a patient, wherein the individual patient data of the neural status indicates brain regions affected by a neural event;
collecting additional data from the patient using functional magnetic resonance imaging (fMRI), positron emission tomography (PET), single-photon emission computed tomography (SPECT), or a combination thereof;
generating from the additional data, a therapeutic brain network response map of a treatment, wherein the therapeutic brain network response map indicates brain responses to the treatment, wherein generating the therapeutic brain network response map comprises aligning the additional data to a template brain, and the therapeutic brain network response map indicates a frequency of activation of brain regions affected by the treatment, average signal values of the brain regions affected by the treatment, or a combination thereof; and
predicting an efficacy of the treatment for the patient based on a comparison of the individual patient data of the neural status and the therapeutic brain network response map by a statistical prediction model for the treatment implemented by a processor executing computer-readable instructions encoded on a non-transitory medium.
|