| CPC A61B 5/0205 (2013.01) [A61B 5/4848 (2013.01); A61B 5/7275 (2013.01); A61M 5/007 (2013.01); A61M 5/1723 (2013.01); A61B 5/28 (2021.01); A61B 5/296 (2021.01); A61M 2205/33 (2013.01); G16H 50/30 (2018.01)] | 21 Claims |
|
1. A system comprising:
at least one processor programmed and/or configured to:
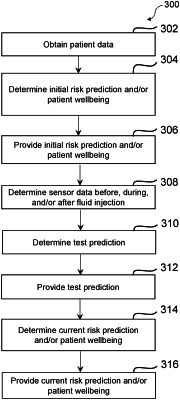
obtain patient data associated with a patient;
determine, based on the patient data, an initial risk prediction for the patient associated with a fluid injection to be administered to the patient, wherein the initial risk prediction includes a probability that the patient experiences at least one adverse event in response to the fluid injection, and wherein the at least one adverse event includes: an extravasation, a post-contrast acute kidney injury, an acute adverse event, a contrast media induced nephrotoxicity, and a thyrotoxicosis such that the initial risk prediction includes a probability that the patient experiences the extravasation, a probability that the patient experiences the post-contrast acute kidney injury, a probability that the patient experiences the acute adverse event, a probability that the patient experiences the contrast media induced nephrotoxicity, and a probability that the patient experiences the thyrotoxicosis;
provide, to a user device, before the fluid injection is administered to the patient, the initial risk prediction, wherein the initial risk prediction further includes at least one of: a prompt to administer a medication to the patient before the fluid injection, a prompt to adjust an injection protocol for the fluid injection, a prompt to adjust an imaging protocol for an imaging scan, a prompt to prepare the patient before the fluid injection, a prompt to observe and/or follow-up with the patient after the fluid injection, or any combination thereof;
obtain sensor data associated with the patient and determined after the fluid injection is started;
determine, based on the sensor data determined after the fluid injection is started, a current risk prediction for the patient associated with the fluid injection, wherein the current risk prediction includes a probability that the patient experiences the at least one adverse event in response to the fluid injection; and
provide, to the user device, the current risk prediction.
|