| CPC A61B 18/1492 (2013.01) [A61B 5/287 (2021.01); A61B 5/6858 (2013.01); A61B 5/0538 (2013.01); A61B 5/6885 (2013.01); A61B 2018/0016 (2013.01); A61B 2018/00208 (2013.01); A61B 2018/00267 (2013.01); A61B 2018/00357 (2013.01); A61B 2018/00577 (2013.01); A61B 2018/1407 (2013.01); A61B 2018/1417 (2013.01); A61B 2018/1467 (2013.01); A61B 2018/1475 (2013.01); A61B 2562/0271 (2013.01); A61B 2562/04 (2013.01); A61B 2562/046 (2013.01); A61N 1/056 (2013.01)] | 40 Claims |
|
1. A medical device system comprising:
a structure comprising a plurality of elongate members; and
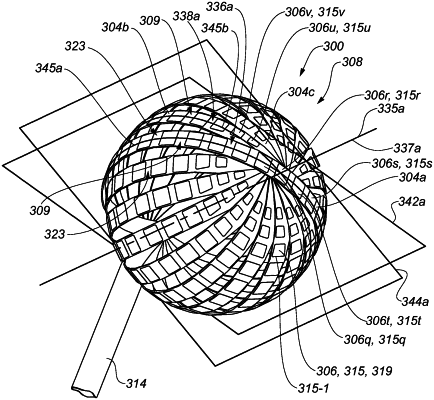
a plurality of electrodes positionable in a bodily cavity and supported by the plurality of elongate members of the structure, the plurality of elongate members including a first elongate member and a second elongate member,
wherein the structure is selectively moveable between:
a delivery configuration in which the structure is sized to be percutaneously deliverable to the bodily cavity, and
a deployed configuration in which the structure is sized too large to be percutaneously deliverable to the bodily cavity, and in which a first portion of the first elongate member is in an at least partially overlapping relationship with a first portion of the second elongate member,
wherein a first group of electrodes of the plurality of electrodes is arranged in a first ringed arrangement about an axis of the structure in a state in which the structure is in the deployed configuration,
wherein the first group of electrodes comprises a first electrode supported by the first portion of the first elongate member and comprises a second electrode supported by the first portion of the second elongate member, the first electrode including an exterior surface having a different size, shape, or both size and shape than a corresponding exterior surface of the second electrode, such that the first ringed arrangement in which the first group of electrodes is arranged comprises electrodes including exterior surfaces having different sizes, shapes, or both sizes and shapes,
wherein the different size, shape, or both size and shape of the exterior surface of the first electrode compared to the corresponding exterior surface of the second electrode is or are configured to accommodate the at least partially overlapping relationship between the first portion of the first elongate member and the first portion of the second elongate member, and
wherein each electrode of at least one electrode in the first group of electrodes is configured to selectively transmit energy from the exterior surface of the electrode, the energy sufficient to ablate tissue.
|