| CPC A61K 33/00 (2013.01) [A61K 9/0053 (2013.01); A61K 9/0056 (2013.01); A61K 9/20 (2013.01); A61K 9/2009 (2013.01); A61K 9/2013 (2013.01); A61K 31/195 (2013.01); A61K 31/198 (2013.01); A61K 31/375 (2013.01); A61K 31/714 (2013.01); A61K 36/21 (2013.01); A61K 36/734 (2013.01); A61K 38/44 (2013.01); A61K 45/06 (2013.01); C12Y 107/02001 (2013.01)] |
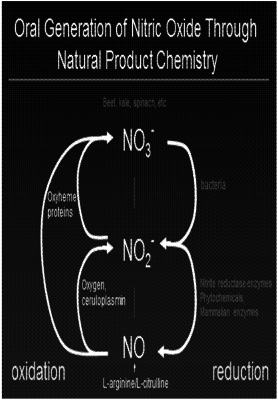
| AS A RESULT OF REEXAMINATION, IT HAS BEEN DETERMINED THAT: |
| Claims 1, 4 and 14 are determined to be patentable as amended. |
| Claims 2, 3, 5-13, and 15-27, dependent on an amended claim, are determined to be patentable. |
| New claims 28-32 are added and determined to be patentable. |
|
1. A composition comprising:
wherein the composition is an oral composition [ in a single dosage form ] and wherein the composition comprises
[ wherein a range of nitrite: nitrate in the composition is 1:0.1-1.0.]
|
|
4. The composition of claim 1, wherein the composition further comprises
|
|
14. The composition of claim 1, wherein the composition is in a form of a lozenge, tablet, [ or ] wafer
|
|
28. [ A method of increasing levels of nitric oxide in an individual, the method comprising:
ingesting a composition only once or twice per day, the composition comprising:
1 mg to 150 mg of a nitrite salt and
10 mg to 10,000 mg of nitrite reductase,
wherein the composition is an oral composition in a single dosage form and wherein the composition comprises 1,000 mg to 10,000 mg of beet root in powder form.]
|
|
29. [ The method of claim 28, wherein the individual ingesting the composition is an adult human.]
|
|
30. [ The method of claim 28, wherein the composition is only ingested once per day.]
|
|
31. [ The method of claim 30, wherein the individual ingesting the composition is an adult human.]
|
|
32. [ A method of increasing levels of nitric oxide in an individual, the method comprising:
ingesting a composition, the composition comprising:
1 mg to 150 mg of a nitrite salt and
10 mg to 10,000 mg of nitrite reductase,
wherein the composition is an oral composition in a single dosage form and wherein the composition comprises 1,000 mg to 10,000 mg of beet root in powder form;
wherein the individual ingesting the composition has a bodyweight between 70 and 80 kilograms.]
|