| CPC H01M 4/628 (2013.01) [C08F 259/04 (2013.01); C08F 271/00 (2013.01); H01M 4/663 (2013.01); H01M 8/0239 (2013.01); H01M 8/0245 (2013.01); H01M 8/188 (2013.01); C08F 2800/20 (2013.01); C08F 2810/20 (2013.01); H01M 2004/021 (2013.01); H01M 2004/028 (2013.01)] | 13 Claims |

|
1. A battery, comprising
an anode,
a cathode,
an electrolyte disposed between the anode and the cathode,
a halogen in contact with the cathode, wherein the cathode comprises a cathode active layer, and
a metal in contact with the anode,
wherein a polymeric halogen sequestering agent (HSA) is distributed in the cathode active layer, and wherein the polymeric HSA is a polymer or co-polymer comprising a monomer of Formula (I):
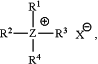 wherein
Z is N, P or S,
R1 is allyl or vinyl,
R2 is selected from the group consisting of allyl, vinyl, and optionally substituted branched or unbranched C1 to C18 alkyl, and X− is Cl−, I−, Br−, F−, SCN−, OCN−, OH−, C2O42−, HCOO−, HCO3−, CO32−, OCl−, OBr−, BrO3−, ClO3−, SO32−, NO2−, IO3−, H2PO4−, HPO42−, SO4−, NO3−, ClO4, bis(trifluoromethylsulfonyl)-imide, bis(fluorosulfonyl)imide, hexafluorophosphate, tetrafluoroborate, tris(pentafluoro)trifluorophosphate, acetate, propionate, pentanoate, hexanoate, or a combination thereof, or
R2 and X− are absent, and
R3 and R4 are each independently selected from allyl, vinyl, optionally substituted branched or unbranched C1 to C18 alkyl, or
R3 and R4 are joined to form a 4, 5, or 6-membered ring together with Z, optionally comprising one or more heteroatoms selected from the group consisting of O, P and N, wherein said ring is optionally substituted,
wherein each optional substituent is independently selected from the group consisting of allyl, vinyl, branched and unbranched C1 to C18 alkyl, Cl, Br, I, F, —OR5, —NR5R6, —N+R5R6R7, —SR5, —COOR5, and carbonyl,
wherein R5, R6 and R7 are each independently selected from branched or unbranched C1 to C18 alkyl or H, and any two of R5, R6 and R7 are optionally joined to form a 4, 5, or 6-membered ring together with N.
|