| CPC G16H 40/40 (2018.01) [G06F 21/105 (2013.01); G16H 20/17 (2018.01); G16H 40/20 (2018.01); G16H 40/63 (2018.01); G16H 40/67 (2018.01); G06F 21/1075 (2023.08); G06F 21/12 (2013.01); G06F 21/121 (2013.01)] | 20 Claims |
|
1. A medical device system, comprising:
at least one processor; and
a memory storing instructions which, when executed by the at least one data processor, result in operations comprising:
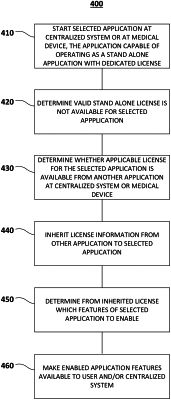
receiving, from a centralized system coupled to a plurality of medical devices associated with a site, at least one request for a first license for a first medical device application and a second license for a second medical device application, wherein at least one of the plurality of medical devices comprises the first medical device application, and wherein at least one of the plurality of medical devices comprises the second medical device application;
generating, after receiving the at least one request, the first license and the second license such that a portion of the first license includes an applicable license to operate at least one feature of the second medical device application, wherein the portion of the first license forms an inherited license, and wherein the first license and the second license are assigned to the site;
transmitting, to the centralized system, the generated first license and the generated second license; and
causing a first medical device to start the second medical device application with the at least one feature.
|