| CPC G16H 10/60 (2018.01) [G16B 20/00 (2019.02); G16B 50/20 (2019.02); G16H 10/40 (2018.01)] | 19 Claims |
|
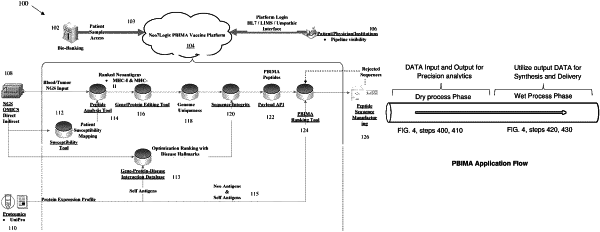
1. A precision-based immunomolecular augmentation (PBIMA) computerized method for designing and treating a patient with a customized therapeutic peptides or peptide vaccine, comprising:
receiving a data input, by a cloud-based system, of a patient data comprising one or more of: a patient transcriptomics data, and a patient urine proteomics data;
computing a precision data output, by the cloud-based system, of a vaccine composition comprising a plurality of ranked peptide sequences encoding self-antigens and/or neo-antigens for a CD4+/CD8+ natural killer (NK) cell modulation specific to a patient's profile, and able to elicit an effective therapeutic response against a patient disease;
computing a CRISPR prime editing and an intracellular multi-core processing on the vaccine composition to produce a DNA-RNA and epigenetic modulation plurality of immunopeptide sequences;
conducting an immunopeptide synthesis and manufacturing of the vaccine composition;
conducting the delivery of the vaccine composition to a patient's clinician or institution, and administrating the vaccine composition to the patient; and
wherein the patient has been diagnosed with, and/or is genetically predisposed to one or more diseases comprising: a cancer, an autoimmune disease, a neurodegenerative disease, and/or a pathogenic infectious disease.
|