| CPC G01N 19/00 (2013.01) [A61B 5/021 (2013.01); A61L 15/32 (2013.01); A61B 2090/064 (2016.02); A61F 2240/008 (2013.01); A61K 49/00 (2013.01); A61L 2400/04 (2013.01)] | 16 Claims |
|
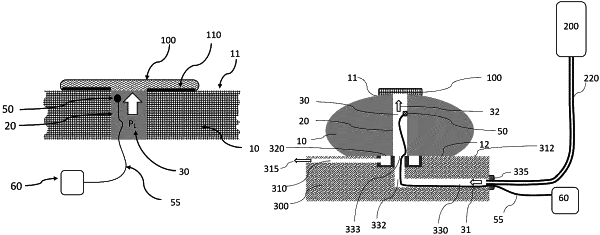
1. A system for ex vivo testing performance of a hemostatic or sealing product attached to an animal organ and fully covering a cored channel in said organ, comprising:
a) a pressure sensor positioned proximate to said hemostatic or sealing product in said cored channel;
b) a monitoring or recording device configured to receive pressure readings from said pressure sensor; and
c) a pressurized fluid source connected to said cored channel and configured to supply said pressurized fluid into said cored channel under constant or variable pressure.
|