| CPC C07D 498/04 (2013.01) [C07D 211/94 (2013.01); C07D 213/64 (2013.01); C07D 213/69 (2013.01); C07D 213/70 (2013.01); C07D 213/73 (2013.01); C07D 213/74 (2013.01); C07D 217/24 (2013.01); C07D 237/14 (2013.01); C07D 239/54 (2013.01); C07D 239/56 (2013.01); C07D 241/20 (2013.01); C07D 401/04 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/04 (2013.01); C07D 405/04 (2013.01); C07D 405/06 (2013.01); C07D 405/12 (2013.01); C07D 409/04 (2013.01); C07D 413/04 (2013.01); C07D 413/06 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01); C07D 491/048 (2013.01); C07D 495/04 (2013.01)] | 14 Claims |
|
1. A method of treating prostate cancer in a subject in need thereof comprising administering to the subject a therapeutically effective amount of compound having the structure of Formula (I), or a pharmaceutically acceptable salt thereof,
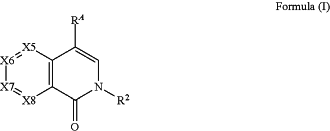 wherein:
R2 is selected from CH3, CH2CH3, CH2CF3, CH2F, CHF2, CF3, CH2D, CHD2, or CD3;
X5 is C—R5 or N;
X6 is C—R6 or N;
X7 is C—R7 or N;
X8 is C—R8 or N; wherein no more than two of X5, X6, X7, or X8 may be N;
R5 is hydrogen, halogen, —CN, —NHR61, —N(R61)2, alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroarylalkyl, wherein each R61 is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroarylalkyl;
R6 is hydrogen, halogen, —CN, —NHR61, —N(R61)2, alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroarylalkyl, wherein each R61 is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroarylalkyl;
R7 is hydrogen, halogen, —CN, —NHR61, —N(R61)2, alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroarylalkyl, wherein each R61 is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroarylalkyl;
R8 is hydrogen, halogen, or alkyl;
RA is
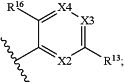 X2 is N or C—R12, wherein R12 is hydrogen, halogen, alkyl, or alkoxy;
R13 is —Y—Z;
Y is selected from a bond, —CH2—, or —CH(C1-C4 alkyl)-;
Z is selected from —SO2R21, —N(R22)SO2R21, —SO2N(R22)2, —N(R22)SO2N(R22)2, —CON(R22)2, —N(R22)CO2R21, —N(R22)CON(R22)2, —N(R22)COR21, —COR21, —OC(O)N(R22)2, —OSO2N(R22)2, or —N(R22)SO3R21;
X3 is N or C—R14, wherein R14 is hydrogen, halogen, —CN, alkyl, cycloalkyl, or alkoxy;
X4 is N or C—R15, wherein R15 is hydrogen, halogen, alkyl, —CN, or alkoxy;
R16 is hydrogen, halogen, —N(H)COX, or —W—X, wherein W is a bond, —O—, —S—, or —NH—, and X is selected from alkyl, aryl, aralkyl, cycloalkyl, cycloalkylalkyl, alkynyl, cycloalkylalkynyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroarylalkyl;
each R21 is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroarylalkyl; and
each R22 is independently selected from hydrogen, alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, or heteroarylalkyl.
|