| CPC C07D 487/04 (2013.01) [A61K 9/1635 (2013.01); A61K 9/1652 (2013.01); A61K 31/4162 (2013.01); C07D 491/18 (2013.01); C07D 513/04 (2013.01); C07D 519/00 (2013.01); C07F 9/6561 (2013.01); C07H 15/26 (2013.01); C07B 2200/13 (2013.01)] | 11 Claims |
|
1. A compound of formula (I):
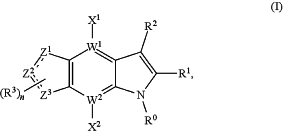 a tautomer, a pharmaceutically acceptable salt, or a deuterated derivative thereof;
wherein:
(i) R0 is
(a) a C1-C8 linear, branched, or cyclic alkyl group, wherein the alkyl group is optionally substituted with 1-4 RA; or
(b) a 5- to 14-membered aromatic ring optionally substituted with 1-4 RA;
wherein each RA is independently a halogen, cyano, hydroxy, thiol, sulfonic acid, sulfonamide, sulfinamide, amino, amide, carboxylic acid, 5- to 10-membered aromatic ring, or a C1-C6 linear, branched, or cyclic group,
wherein the amide nitrogen atom in the amide of RA is optionally substituted with a heterocyclyl group that is optionally further substituted with oxo,
wherein each C1-C6 linear, branched, or cyclic group is, independently, an alkyl, alkoxy, thioalkyl, alkylsulfoxide, alkylsulfonyl, alkylsulfonamide, alkylsulfinamide, aminoalkyl, or alkylamide,
wherein each 5- to 10-membered aromatic ring or C1-C6 linear, branched, or cyclic group, independently, is optionally substituted with 1-4 substituents, wherein each substituent, independently, is a halogen, a C1-C6 linear, branched, or cyclic group, or a methoxy, or
wherein an RA group is optionally linked to an RB group or to an R2 group;
(ii) R1 is
(a) a hydrogen,
(b) a C1-C8 linear, branched, or cyclic alkyl group, wherein the alkyl group is optionally substituted with 1-4 substituents, wherein each substituent, independently, is a
halogen,
cyano,
cyanoalkyl,
hydroxy,
alkylsulfonyl, or
C1-C6 linear, branched, or cyclic group, wherein the C1-C6 linear, branched, or cyclic group is an alkyl or alkoxy group, and wherein the C1-C6 linear, branched, or cyclic group is optionally substituted with 1-4 substituents, wherein each substituent, independently, is a
halogen,
hydroxy, or
C1-C6 linear, branched, or cyclic alkoxy group,
(c) a C1-C8 linear, branched, or cyclic alkoxy or cyclic thioalkyl group optionally substituted with 1-4 substituents, wherein each substituent independently is a
halogen,
cyano,
cyanoalkyl;
sulfone,
sulfonamide,
hydroxy, or
a C1-C6 linear, branched, or cyclic alkyl group optionally substituted with 1-4 halogens or alkoxy groups;
(d) a C1-C6 linear, branched, or cyclic alkylsulfonyl group optionally substituted with C1-C6 linear or branched alkyl groups;
(e) an aminosulfonyl group, optionally substituted with 1 or 2 substituents, wherein each substituent independently is a
C1-C6 linear, branched, or cyclic alkyl group;
(f) a C1-C6 linear, branched, or cyclic alkylsulfonyl amino group;
(g) a phosphine oxide group, optionally substituted with 1 or 2 substituents, wherein each substituent, independently, is a
C1-C6 linear, branched, or cyclic alkyl group;
(h) a C1-C6 linear, branched, or cyclic trialkylsilyl group; or
(i) a C1-C6 alkylamide;
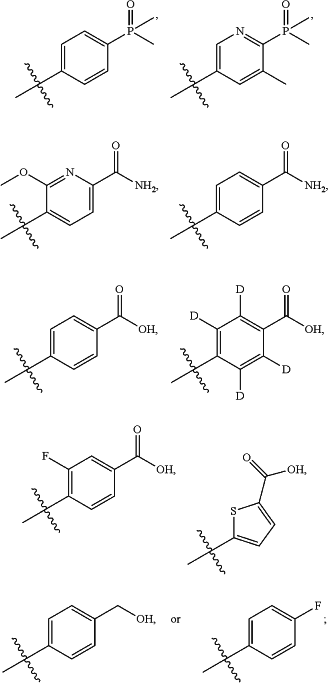
(iii) R2 is
 (iv) each of X1 and X2, independently, is a hydrogen, halogen, cyano, hydroxy, C1-C6 linear, branched, or cyclic group, wherein each C1-C6 linear, branched, or cyclic group, independently, is an alkyl, alkoxy, thioalkyl, or aminoalkyl group, and wherein each C1-C6 linear, branched, or cyclic group is optionally substituted by 1-4 independently chosen halogens;
(v) each of W1 and W2 independently is a C or N;
(vi) each
 is a single or double bond, provided that no more than one
 is a double bond;
(vii) each R3 independently, is a hydrogen, halogen, cyano, C1-C6 linear, branched, or cyclic alkyl group, or C1-C6 linear, branched, or cyclic alkoxy group, wherein the C1-C6 linear, branched, or cyclic alkyl group and the C1-C6 linear, branched, or cyclic alkoxy group is optionally substituted with 1-4 substituents, wherein each substituent, independently, is a halogen, hydroxy or carboxylic acid;
(viii) n is 0, 1, 2, or 3; and
(ix) two of Z1, Z2, and Z3 are nitrogen, and the third is carbon or nitrogen, wherein the valences of carbon or nitrogen are completed with hydrogen, halogen, C1-C6 linear, branched, or cyclic alkyl groups, or C1-C6 linear, branched, or cyclic alkoxy groups, wherein the C1-C6 linear, branched, or cyclic alkyl groups and the C1-C6 linear, branched, or cyclic alkoxy groups are optionally substituted with 1-4 substituents, wherein each substituent, independently, is halogen, hydroxy, or carboxylic acid.
|