| CPC C07D 273/04 (2013.01) [B01J 31/1658 (2013.01); B01J 31/2217 (2013.01); C07B 53/00 (2013.01); C07C 67/31 (2013.01); B01J 2231/70 (2013.01); B01J 2531/0252 (2013.01); B01J 2531/48 (2013.01); C07B 2200/07 (2013.01); C07C 2602/08 (2017.05)] | 10 Claims |
|
1. A preparation method for a high optical indoxacarb intermediate (2S)-5-chloro-2,3-dihydro-2-hydroxy-1-oxo-1H-indene-2-carboxylic acid methyl ester, wherein an asymmetric synthesis is catalyzed by a chiral Zr-salen polymer, a method of producing the chiral Zr-salen polymer, comprising the steps of:
(1) adding a solvent I, 3-tert-butyl-5-styrylsalicylaldehyde and (1S,2S)-(−)-1,2-diphenylethylenediamine or (1S,2S)-(+)-1,2-cyclohexanediamine into a reaction flask, raising the temperature for reflux reaction, after the reaction is finished, a condensate is obtained after lowering the temperature and filtration, wherein
the solvent I is methanol;
(2) dissolving the condensate in a solvent II, raising the temperature to 50-60° C., and dripping a reducing agent, removing the solvent II after the reaction, adding water into the system, and a ligand monomer of catalyst A or catalyst B is obtained after extraction, desolvation, crystallization, lowering the temperature and filtration, wherein
the solvent II is tetrahydrofuran;
(3) putting the ligand monomer of catalyst A or catalyst B into a pressure-resistant reaction flask, adding a solvent III and using azobisisobutyronitrile (AIBN) as an initiator, carrying out hydrothermal polymerization at 110-120° C., then a polymer ligand is obtained after filtration and drying, wherein
the solvent III is a mixture of ethanol and water;
(4) putting the polymer ligand and a zirconium-containing metal compound in toluene at 50-80° C. to carry out complexation reaction, after the reaction, a toluene system of catalyst A or catalyst B is obtained, wherein
the zirconium-containing metal compound is selected from zirconium hydroxide, zirconyl chloride octahydrate, zirconium dioxide, zirconium(IV) acetylacetonate, zirconium trifluoroacetylacetonate, n-propyl zirconate, zirconium(IV) tert-butoxide, zirconium(IV) hydrogenphosphate, zirconium(IV) bromide, and mixtures thereof;
and the preparation reaction equation is as follows:
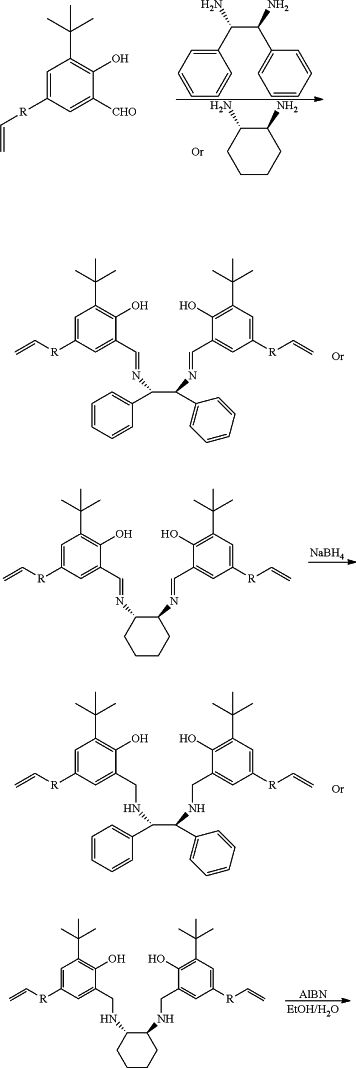 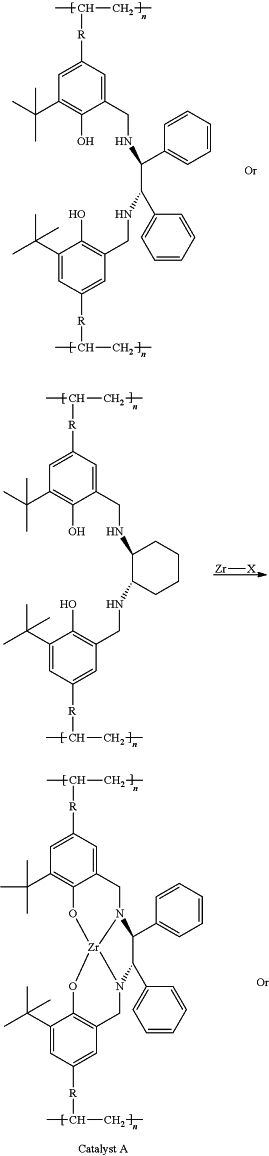 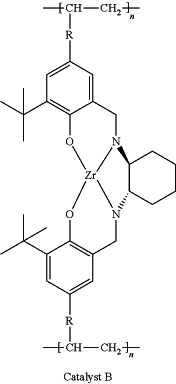 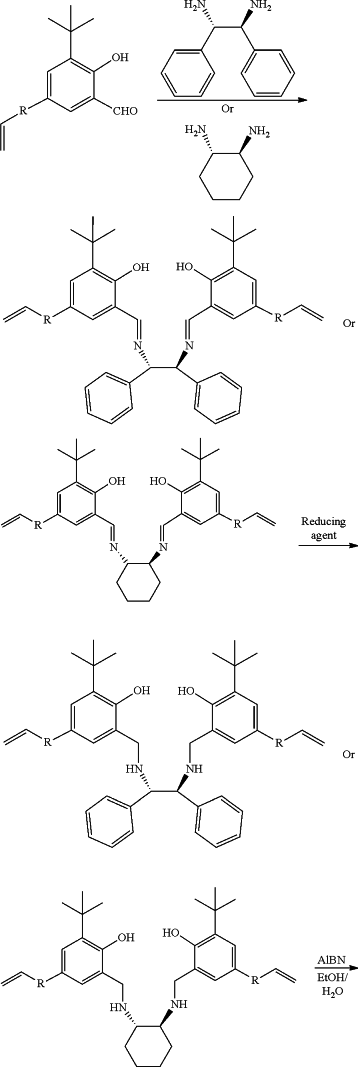 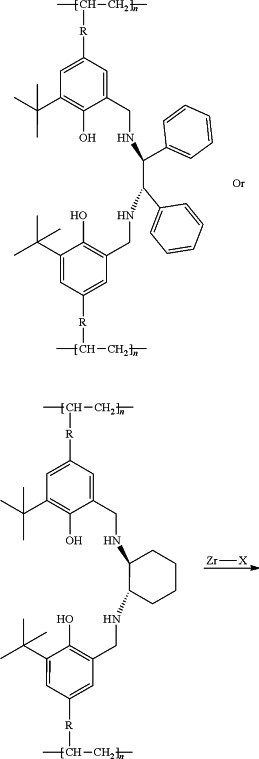 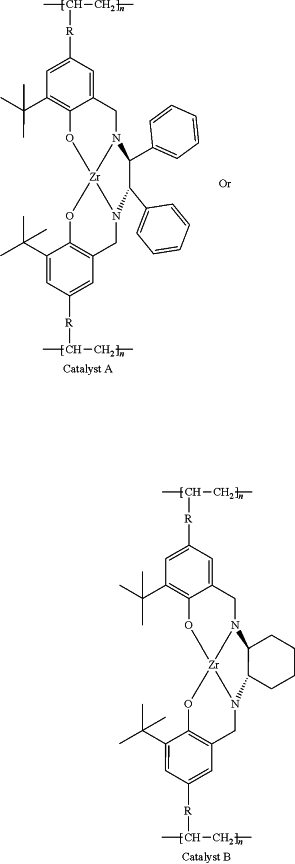 wherein: R is
 n is 100˜20000, and Zr—X is the zirconium-containing metal compound.
|