| CPC C07C 49/743 (2013.01) [C07C 33/12 (2013.01); C07C 59/62 (2013.01); C07D 307/42 (2013.01); C12P 7/38 (2013.01); C12P 7/62 (2013.01)] | 9 Claims |
|
1. A process for preparing compound of Formula 4:
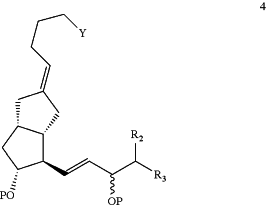 wherein Y is —CH2OP or —COOR1; P is H or a hydroxyl protective group; R1 is C1-7-alkyl, aryl or aralkyl, each of which is unsubstituted or substituted by C1-4-alkyl, C2-7-alkenyl, C2-7-alkynyl, nitro, halogen or alkoxy; R2 is H or C1-4-alkyl; and R3 is C1-7-alkyl, C2-7-alkynyl, aryl or aryloxy, each of which is unsubstituted or substituted by C1-4-alkyl, halogen, or trihalomethyl, the process comprising the steps of:
(1) reacting a starting compound of Formula 1:
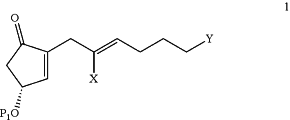 wherein X is F, Cl, Br, I, or —OTs; P1 is a hydroxyl protective group; and Y is as defined above, with a starting cuprate derived from a compound of Formula L1, Formula L2, or Formula L3:
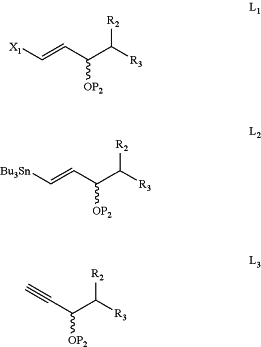 wherein X1 is Cl, Br, or I; P2 is a hydroxyl protective group; and R2 and R3 are as defined above, to form a compound of Formula 2:
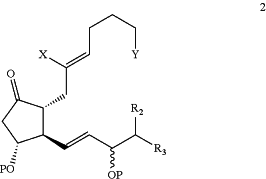 wherein P, X, Y, R2 and R3 are as defined above;
(2) methylenation of a ketone radical of the compound of Formula 2 to form a compound of the following formula 3:
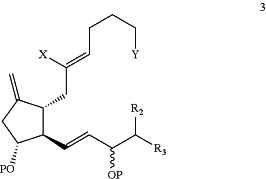 wherein P, X, Y, R2 and R3 are as defined above;
(3) performing an intramolecular cyclization reaction to the compound of Formula 3 to form the compound of Formula 4:
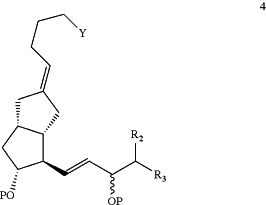 wherein P, Y, R2 and R3 are as defined above; and
(4) optionally performing a deprotecting reaction for removing P1 and/or P2.
|