| CPC C02F 1/46109 (2013.01) [C02F 2001/46133 (2013.01); C02F 2101/12 (2013.01); C02F 2103/08 (2013.01)] | 20 Claims |
|
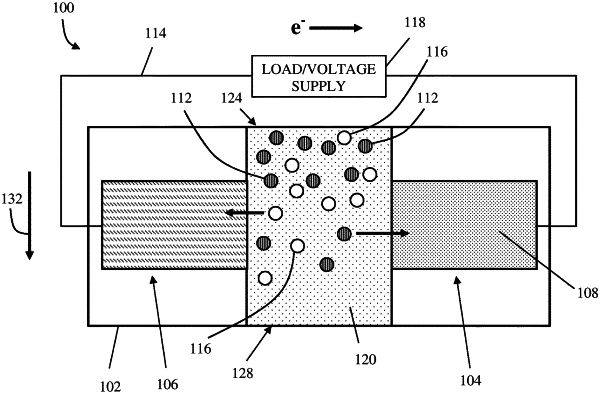
6. A device for removing chloride-containing salts from water, comprising:
a container configured to contain a saline water solution having a first concentration c1 of chloride ions;
a first electrode arranged in fluid communication with the saline water solution, the first electrode including a conversion material that is insoluble in the saline water solution, the conversion material having a composition that includes at least two or more of aluminum, chlorine, copper, iron, oxygen, and potassium, the composition variable over a range with respect to a quantity of chloride ions per formula unit; and
a power source configured to supply current to the first electrode so as to induce a reversible conversion reaction in which the conversion material (i) associates with the chloride ions in the saline water solution in a first operating state to generate a treated water solution having a second concentration c2 of the chloride ions and (ii) dissociates the chloride ions therefrom into the saline water solution in a second operating state to generate a wastewater solution having a third concentration c3 of the chloride ions where c3>c1>c2, wherein the conversion material is represented by the formula AlCuClx, where 0≤x≤4.
|