| CPC B65B 1/28 (2013.01) [B65B 1/06 (2013.01); B65B 9/12 (2013.01); B65B 37/02 (2013.01); B65B 39/001 (2013.01); B65G 69/181 (2013.01)] | 12 Claims |
|
1. A method for transferring a biological or pharmaceutical product from a dispensing container to a receiving container comprising:
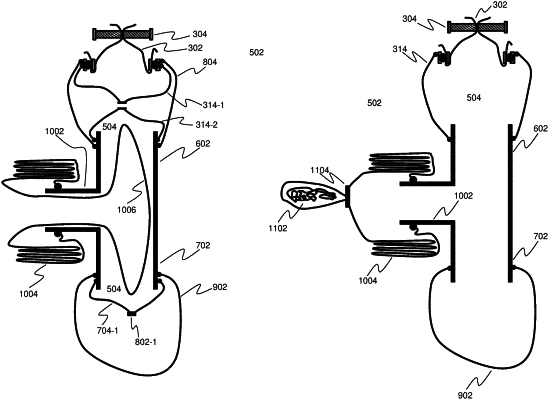
flowing a biological or pharmaceutical product from a dispensing container through a sealed conduit to a receiving container, the sealed conduit including a rigid section and a flexible feed sleeve that extends from the dispensing container to the rigid section of the conduit, wherein the biological or pharmaceutical product flows through the flexible feed sleeve into the livid section of the conduit, the rigid section being lined by a flexible conduit liner, the flexible conduit liner including a first end and a second and opposite end, the flexible conduit liner preventing the biological or pharmaceutical product from contacting a surface of the rigid section;
containing residual biological or pharmaceutical product within the flexible conduit liner after flow of the biological or pharmaceutical product has ceased;
removing the flexible conduit liner from the conduit without releasing the contained residual biological or pharmaceutical product from within the flexible conduit liner; and
wherein the method further includes the steps of:
after flow of the biological or pharmaceutical product has ceased, crimping the used flexible feed sleeve at an intermediate section and separatism the flexible teed sleeve into two parts wherein each part includes a sealed end where the flexible feed sleeve as been crimped;
inserting a second and unused flexible feed sleeve between the two separate parts of the used flexible teed sleeve and extending the second flexible feed sleeve over each part of the used flexible feed sleeve such that the second flexible feed sleeve extends from the dispensing container to the rigid section of the conduit; and
removing the separated parts of the used flexible feed sleeve from within the second flexible feed sleeve without permitting release of a residual biological or pharmaceutical product.
|