| CPC A61N 1/3968 (2013.01) [A61N 1/046 (2013.01); A61N 1/0476 (2013.01); A61N 1/0484 (2013.01); A61N 1/3987 (2013.01); A61N 1/3993 (2013.01); A61N 1/3925 (2013.01)] | 23 Claims |
|
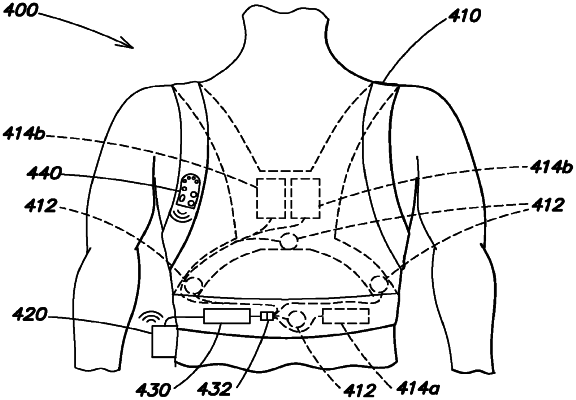
1. A wearable medical device, comprising:
a waterproof harness configured to be worn about a torso of a patient;
a plurality of ECG sensing electrodes configured to detect the patient's ECG and configured to attach to the harness at varying positions;
a plurality of therapy electrodes configured to provide at least one biphasic shock to the patient and configured to attach to the harness such that the plurality of therapy electrodes are disposed on two sides of the patient's torso when the harness is worn by the patient;
a control unit in electronic communication with the plurality of ECG sensing electrodes and the plurality of therapy electrodes and configured to monitor the detected ECG of the patient and, in response to detecting a cardiac arrhythmia of the patient, provide the at least one biphasic shock, the control unit comprised within a waterproof case;
a water-resistant or waterproof connection pod that is configured to electrically couple the plurality of ECG sensing electrodes, the plurality of therapy electrodes, and the control unit, wherein the connection pod provides the detected ECG to the control unit and a biphasic shock current to the plurality of therapy electrodes; and
a user interface device in electronic communication with the control unit, wherein the user interface device comprises at least one patient responsiveness button by which the patient can indicate that the patient is conscious.
|