| CPC A61M 16/024 (2017.08) [A61B 5/0816 (2013.01); A61B 5/6844 (2013.01); A61M 16/0069 (2014.02); A61M 16/0672 (2014.02); A61M 16/161 (2014.02); A61M 2016/003 (2013.01); A61M 2016/0027 (2013.01); A61M 2202/0208 (2013.01); A61M 2205/3365 (2013.01); A61M 2230/42 (2013.01); A61M 2230/432 (2013.01)] | 17 Claims |
|
1. A respiratory system configured to deliver a respiratory therapy to a patient, the system also configured to provide information related to the patient, the system comprising:
a respiratory device configured to deliver nasal high flow therapy, the respiratory device comprising a controller, wherein the controller is configured to:
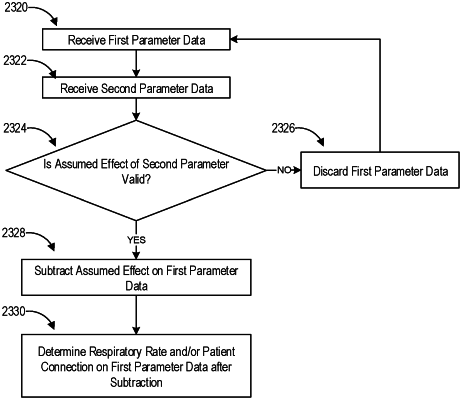
receive measurements of a first parameter of a flow of gases or representative of performance of a component of the respiratory device, wherein the first parameter is a flow rate;
receive measurements of a second parameter of a gases flow or representative of performance of a component of the respiratory device, wherein the second parameter has an assumed effect on the first parameter wherein the first parameter is calculated independent of the second parameter;
determine whether the assumed effect is valid; and
discard the first parameter from a validated first parameter dataset in response to the assumed effect being invalid, the controller configured to use the validated first parameter dataset to estimate a respiratory rate of the patient.
|