| CPC A61K 47/548 (2017.08) [A61K 47/6803 (2017.08); A61K 47/6849 (2017.08); A61K 47/6889 (2017.08); C07F 9/3839 (2013.01); C07F 9/40 (2013.01); A61P 29/00 (2018.01); A61P 35/00 (2018.01)] | 20 Claims |
|
1. A method for treating a disease or disorder by providing to a subject having the disease or disorder a composition comprising a compound having formula (III)
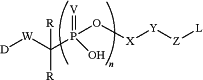 wherein V is selected from O and S; W is selected from a bivalent, straight or branched, saturated or unsaturated, optionally substituted C1-30 hydrocarbon chain wherein one or more methylene units are optionally and independently replaced by —O—, —S—, —N(R)—, —C(O)—, C(O)O—, OC(O)—, —N(R)C(O)—, —C(O)N(R)—, —S(O)—, —S(O)2-, —N(R)SO2-, SO2N(R)—, a heterocyclic group, an aryl group, or a heteroaryl group;
X is a tuning element selected from a covalent bond; a carbon atom; a heteroatom; an optionally substituted group selected from the group consisting of acyl, aliphatic, heteroaliphatic, aryl, heteroaryl, and heterocyclic; nucleoside, protease sensitive group, cathepsin B sensitive group, or glycosidase sensitive group;
Y is selected from a covalent bond or a bivalent, straight or branched, saturated or unsaturated, optionally substituted C1-30 hydrocarbon chain wherein one or more methylene units are optionally and independently replaced by —O—, —S—, —N(R)—, —C(O)—, C(O)O—, OC(O)—, —N(R)C(O)—, —C(O)N(R)—, —S(O)—, —S(O)2-, —N(R)SO2-, SO2N(R)—, a heterocyclic group, an aryl group, or a heteroaryl group;
D is a payload;
Z is a linkage formed between (i) a reactive functional group selected from the group consisting of N-hydroxysuccinimide, para-nitrophenyl carbonate, para-nitrophenyl carbamate, pentafluorophenyl, haloacetamide, maleimide, hydroxylamine, strained cycloalkyne, heterocycloalkyne, alkyne, diene, azadiene, and heterocyclic azadience, wherein halo is iodine (I), bromine (Br), fluorine (F), or chlorine (Cl) and hetero is N, O, or S, and (ii) an S, NR, or 0 group on L;
each occurrence of R is independently hydrogen, a suitable protecting group, an acyl moiety, arylalkyl moiety, aliphatic moiety, aryl moiety, heteroaryl moiety, or heteroaliphatic moiety;
L is a cell-specific targeting ligand; and n is 1, 2, 3, or 4; and
a pharmaceutically acceptable salt or carrier.
|