| CPC A61K 31/702 (2013.01) [A23L 33/125 (2016.08); A23L 33/135 (2016.08); A61K 31/716 (2013.01); A61K 31/733 (2013.01); A61K 35/741 (2013.01); A61K 45/06 (2013.01); A61P 1/00 (2018.01); A61P 3/02 (2018.01); A61P 35/00 (2018.01); A61P 35/04 (2018.01); A61P 43/00 (2018.01); A23V 2002/00 (2013.01)] | 22 Claims |
|
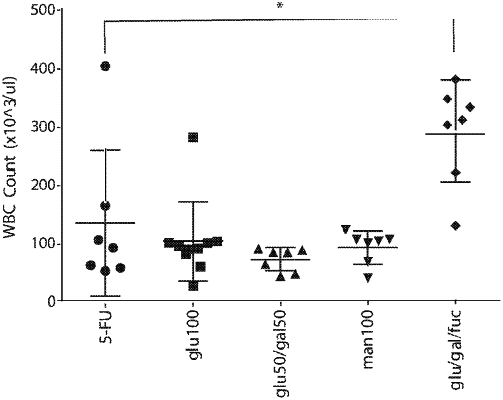
1. A method for reducing inflammation of a gastrointestinal inflammatory disease in a subject having an immune imbalance, comprising administering to the subject an effective amount of a composition comprising a glycan therapeutic preparation, wherein:
i) the glycan therapeutic preparation comprises branched glycans comprising glucose, galactose, arabinose, mannose, fructose, xylose, fucose, or rhamnose glycan units;
ii) the glycan therapeutic preparation comprises an average degree of branching (DB) of at least 0.01;
iii) at least 50% of the glycans in the glycan therapeutic preparation have a degree of polymerization (DP) of at least 3 and less than 30 glycan units; and
iv) the ratio of alpha- to beta-glycosidic bonds present in the glycans of the glycan therapeutic preparation is between about 1:1 to about 5:1; thereby reducing inflammation of the gastrointestinal inflammatory disease in the subject having the immune imbalance.
|