| CPC A61K 31/573 (2013.01) [A61K 9/0014 (2013.01); A61K 9/06 (2013.01); A61K 9/08 (2013.01); A61K 47/10 (2013.01); A61K 47/22 (2013.01); A61K 47/26 (2013.01); A61P 1/00 (2018.01); A61P 17/14 (2018.01)] | 18 Claims |
|
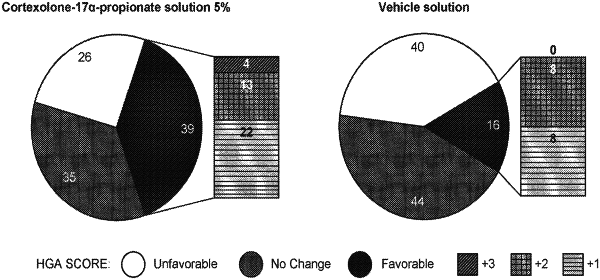
1. A method of treating androgenetic alopecia, the method comprising topically administering to a subject in need thereof, an effective amount of a pharmaceutical formulation comprising:
a) cortexolone-17α-propionate at a concentration of about 5 weight percent to about 15 weight percent,
b) a pharmaceutically acceptable amount of one or more pharmaceutically acceptable solvents, and
c) less than about 5% water,
wherein:
the cortexolone-17α-propionate is fully solubilized in the formulation;
the formulation has a pH between about 4 and about 5; and
wherein the formulation has a storage stability of at least about 6 months at room temperature as measured by the formulation comprising less than 5 weight percent of cortexolone-21-propionate (17α-hydroxy-21-propionyloxy-pregna-4-ene-3,20-dione) or other degradation products.
|