| CPC A61K 31/496 (2013.01) [A61K 31/00 (2013.01); C07D 401/12 (2013.01)] | 20 Claims |
|
1. A process for making a compound of Formula (I) in crystal form:
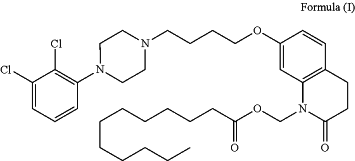 the process comprising the steps of:
(a) dissolving the compound of Formula (I) or a salt or solvate thereof in a first solvent to form a homogeneous drug solution;
(b) optionally combining the drug solution with a second solvent;
(c) cooling the solution; and
(d) further homogenizing the solution to induce formation of the compound of Formula (I) in crystal form.
|