| CPC A61K 31/404 (2013.01) [A61K 45/06 (2013.01); A61P 35/02 (2018.01); C07D 209/08 (2013.01); C07D 209/18 (2013.01)] | 13 Claims |
|
1. A composition comprising a compound of the formula:
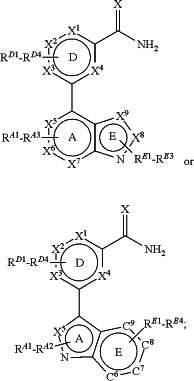 or a salt thereof;
wherein X is S;
wherein each of A, D, and E are aryl or heteroaryl;
wherein each of X1-X4 are independently selected from C and N;
wherein each of X5-X9 are independently selected from C, N, S, and O, when present on a 5-member ring;
wherein each of X5-X9 are independently selected from C and N, when present on a 6-member ring; and
wherein any of the RD1-D4, RA1-A3, and RE1-E4 substituents, when present, are of one of Formulas (IIa-IIq):
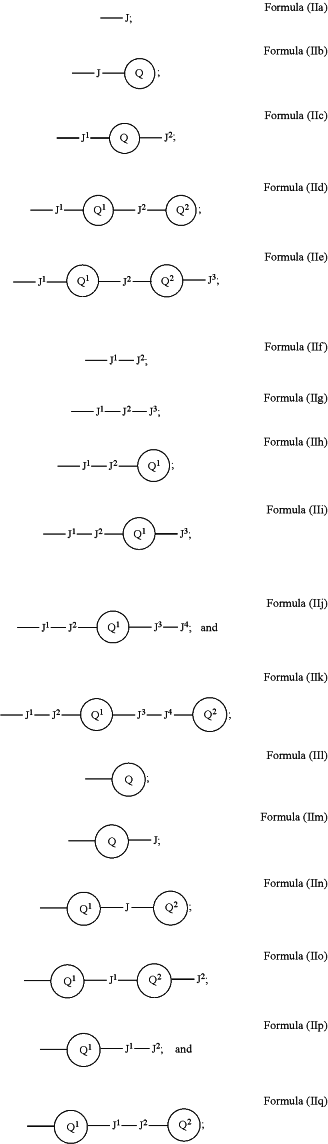 wherein one of J, Q1, or J1, when present, is linked to one of the D, A, or E rings,
wherein each J, J1, J2, J3, and J4, when present, are independently selected from the group consisting of: a covalent bond, H, alkyl1-15, alkenyl1-6, alkynyl1-6, (CH2)0-6C(S)NH2, (CH2)0-6C(O)NH2, O, S, NH, (CH2)0-6C(O)NH(CH2)1-6, (CH2)0-6NHC(O)(CH2)1-6, alkylsulfonyl, sulfonamide, alkylsulfonamide, (CH2)0-6C(S)NH(CH2)1-6, (CH2)0-6O(CH2)1-6, (CH2)0-6OH, (CH2)0-6S(CH2)1-6, (CH2)0-6SH, (CH2)0-6NH(CH2)1-6, (CH2)0-6N(CH2)1-6 (CH2)1-6, (CH2)0-6NH2, (CH2)0-6SO2 (CH2)1-6, (CH2)0-6NHSO2(CH2)1-6, (CH2)0-6 SO2 NH2, halogen, haloalkyl, dihaloalkyl, trihaloalkyl, alkyl with 1-3 halogens at two or more positons along the alkyl, (CH2)1-4SP(Ph)2═S, (CH2)0-6NH(CH2)1-5OH, (CH2)0-6NH(CH2)1-5NH2, (CH2)0-6NH(CH2)1-5SH, (CH2)0-6O(CH2)1-5OH, (CH2)0-6O(CH2)1-5NH2, (CH2)0-6O(CH2)1-5SH, (CH2)0-6S(CH2)1-5OH, (CH2)0-6S(CH2)1-5NH2, (CH2)0-6S(CH2)1-5SH, (CH2)0-6O(CH2)1-6NH(CH2)1-5OH, (CH2)0-6O(CH2)1-6NH(CH2)1-5NH2, (CH2)0-6O(CH2)1-6NH(CH2)1-5SH, (CH2)0-6O(CH2)1-6O(CH2)1-5OH, (CH2)0-6O(CH2)1-6O(CH2)1-5NH2, (CH2)0-6O(CH2)1-6O(CH2)1-5SH, (CH2)0-6O(CH2)1-6 S(CH2)1-5OH, (CH2)0-6O(CH2)1-6S (CH2)1-5NH2, (CH2)0-6O(CH2)1-6 S(CH2)1-5SH, (CH2)0-6S(CH2)1-6NH(CH2)1-5OH, (CH2)0-6S(CH2)1-6NH(CH2)1-5NH2, (CH2)0-6S(CH2)1-6NH(CH2)1-5SH, (CH2)0-6S(CH2)1-6O(CH2)1-5OH, (CH2)0-6S(CH2)1-6O(CH2)1-5NH2, (CH2)0-6S(CH2)1-6O(CH2)1-5SH, (CH2)0-6S(CH2)1-6 S(CH2)1-5OH, (CH2)0-6 S(CH2)1-6 S(CH2)1-5NH2, (CH2)0-6S(CH2)1-6 S(CH2)1-5SH, (CH2)0-6NH(CH2)1-6NH(CH2)1-5OH, (CH2)0-6NH(CH2)1-6NH(CH2)1-5NH2, (CH2)0-6NH(CH2)1-6NH(CH2)1-5SH, (CH2)0-6NH(CH2)1-6O(CH2)1-5OH, (CH2)0-6NH(CH2)1-6O(CH2)1-5NH2, (CH2)0-6NH(CH2)1-6O(CH2)1-5SH, (CH2)0-6NH(CH2)1-6S(CH2)1-5OH, (CH2)0-6NH(CH2)1-6 S(CH2)1-5NH2, (CH2)0-6NH(CH2)1-6S(CH2)1-5SH, (CH2)0-3C(O)O(CH2)0-3, (CH2)0-3C(S)O(CH2)0-3, (CH2)0-3C(O)S(CH2)0-3, (CH2)0-3C(S)S(CH2)0-3, (CH2)0-3C(O)NH(CH2)0-3, (CH2)0-3C(S)NH(CH2)0-3, (CH2)0-3NHC(O)(CH2)0-3, (CH2)0-3NHC(S)(CH2)0-3, (CH2)0-3OC(O)(CH2)0-3, (CH2)0-3OC(S)(CH2)0-3, (CH2)0-3SC(O)(CH2)0-3, (CH2)0-3SC(S)(CH2)0-3(CH2)0-3NHC(O)NH(CH2)0-3, (CH2)0-3NHC(S)NH(CH2)0-3, (CH2)0-3OC(O)NH(CH2)0-3(CH2)0-3OC(S)NH(CH2)0-3, (CH2)0-3SC(O)NH(CH2)0-3, (CH2)0-3SC (S)NH(CH2)0-3(CH2)0-3NHC(O)O(CH2)0-3, (CH2)0-3NHC(S)O(CH2)0-3, (CH2)0-3OC(O)O(CH2)0-3, (CH2)0-3OC(S)O(CH2)0-3, (CH2)0-3SC(O)O(CH2)0-3, (CH2)0-3SC (S)O(CH2)0-3, (CH2)0-3NHC(O)S(CH2)0-3, (CH2)0-3NHC(S)S(CH2)0-3, (CH2)0-3OC(O)S(CH2)0-3, (CH2)0-3OC(S)S(CH2)0-3, (CH2)0-3SC(O)S(CH2)0-3, (CH2)0-3SC (S)S(CH2)0-3, (CH2O)1-6, and trimethyl methane;
wherein each Q, Q1, and Q2, when present, is independently selected from the group consisting of: furan, benzofuran, isobenzofuran, pyrrole, indole, isoindole, thiophene, benzothiophene, benzo[c]thiophene, imidazole, benzimidazole, purine, pyrazole, indazole, oxazole, benzooxazole, isoxazole, benzisoxazole, thiazole, benzothiazole, benzene, napthalene, pyridine, quinolone, isoquinoline, pyrazine, quinoxaline, pyrimidine, quinazoline, pyridazine, cinnoline, phthalazine, thalidomide, triazine, thiadiazole, aziridine, thiirane, oxirane, oxaziridine, dioxirane, azetidine, oxetan, thietane, diazetidine, dioxetane, dithietane, pyrrolidine, tetrahydrofuran, thiolane, imidazolidine, pyrazolidine, oxazolidine, isoxazolidine, thiazolidine, isothiazolidine, dioxolane, dithiolane, piperidine, oxane, thiane, pepierazine, morpholine, thiomorpholine, dioxane, dithiane, trioxane, thithiane, azepane, oxepane, thiepane, homopiperazine, azocane, tetrahydropyran, cyclobutene, cyclopentene, cyclohexene, cycloheptene, 1,3-cyclohexadiene, 1,4-cyclohexadiene, 1,5-cyclooctadiene, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and cycloheptyl;
wherein each Q, Q1, and Q2, when present, may display one or more additional J groups at any position on the Q ring;
wherein RD1-D4, when present are not alkoxy groups;
wherein at least one RE1-E4, when present, is not H;
wherein any alkyl or alkylene groups above may be straight or branched;
wherein any alkyl or alkylene groups above may additionally comprise OH, ═O, NH2, CN, dihaloalkyl, trihaloalkyl, or halogen substituents at one or more carbons;
wherein the number of hydrogens on terminal positions of the groups above may be adjusted if the group is linked to an additional group or if the group is terminal.
|