| CPC A61B 18/04 (2013.01) [A61B 18/02 (2013.01); A61B 18/1492 (2013.01); A61B 18/1815 (2013.01); A61M 25/0084 (2013.01); A61N 1/0551 (2013.01); A61N 1/327 (2013.01); A61N 1/3605 (2013.01); A61N 7/00 (2013.01); A61B 2018/00434 (2013.01); A61B 2018/046 (2013.01); A61M 2025/0089 (2013.01); A61M 2202/048 (2013.01); A61M 2202/049 (2013.01); A61N 2007/003 (2013.01)] | 20 Claims |
|
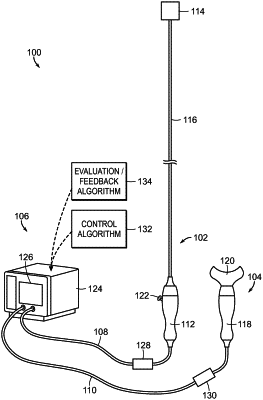
1. A method for treating a human patient with diagnosed systemic sympathetic overactivity, the method comprising:
neuromodulating afferent and efferent renal nerves in the human patient,
wherein neuromodulating afferent and efferent renal nerves includes selectively neuromodulating efferent renal nerves such that a greater percentage of efferent renal nerves are modulated relative to a percentage of modulated afferent renal nerves, and
wherein selectively neuromodulating the efferent renal nerves in the human patient improves a measurable physiological parameter corresponding to the systemic sympathetic overactivity of the human patient.
|