| CPC C07D 471/04 (2013.01) [C07D 401/04 (2013.01); C07D 401/10 (2013.01); C07D 401/14 (2013.01); C07D 405/14 (2013.01); C07D 413/14 (2013.01); C07D 417/14 (2013.01); C07D 453/02 (2013.01); C07D 487/04 (2013.01); C07D 491/08 (2013.01)] | 14 Claims |
|
1. A compound of Formula (I)
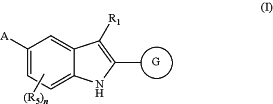 N-oxide, or a salt thereof, wherein:
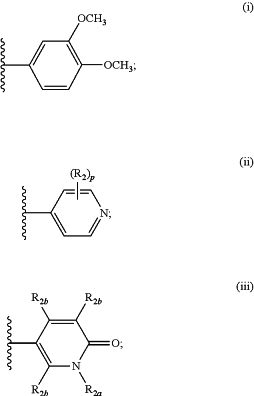
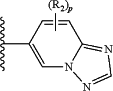
G is:
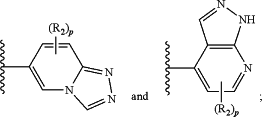
 (iv) a 9-membered heterocyclic ring selected from:
  A is: —CHR12R13, wherein R12 and R13 together with the carbon atom to which they are attached and the hydrogen atom attached to the carbon atom, form a cyclic group selected from azabicyclo[4.1.1]octanyl, azetidinyl, C3-6 cycloalkyl, diazaspiro[4.5]decanonyl, morpholinyl, octahydrocyclopenta[c]pyrrolyl, or quinuclidinyl, each substituted with zero to 3 R12a;
R1 is —CH2CH3 or —CH(CH3)2;
each R2 is independently —CH3 or —OCH3;
R2a is —CH3;
each R2b is independently H or —CH3;
each R12a is independently —OH, —CH3, —CH2CH2CH3, —CH(CH3)2, —CH2CH(CH3)2, —CF3, —CH2CH2CH2CF3, —CH2CN, —CH2C(CH3)2OH, —CH2CH2OCH3, —CH2C(O)NH(CH3), —CH2C(O)N(CH3)2, —CH2C(O)NH2, —CH2CH2S(O)2CH3, —CH2CH2NHS(O)2CH3, —OCH3, —NRxRx, —N(CH3)(CH2CH3), —N(CH3)(CH(CH3)2), —NRx(CH2CHF2)—NH(CH2CF3), —N(CH3)(CH2CH2CF3), —N(CH3)(CH2CH2OCH3), —NH(CH2CN), —N(CH3)CH2N(CH3)2, —NH(CH2C(CH3)2OH), —NH(CH2C(O)NH2), —N(CH3)(OCH3), —NRxCH2CH2S(O)2CH3, —NHC(O)CH3, —NHC(O)CH2CF3, —NHC(O)CHRxNH(CH3), —NRxC(O)CH2N(CH3)2, —NHC(O)CH2N(CH3)(CH2CH3), —NHC(O)CH2N(CH2CH3)2, —NHC(O)CH2NH(CH2C(CH3)2OH), —NHCH2C(O)NRx(CH3), —NHS(O)2CH3, —C(O)C(CH3)3, —C(O)CH(CH2CH3)2, —C(O)CH2OCH3, —C(O)CH2CH2OCH3, —C(O)CH2NH(CH3), —C(O)CH2N(CH3)2, —C(O)CH(CH3)NH(CH3), —C(O)CH2N(CH3)(CH2CH3), —C(O)CH2N(CH2CH3)2, R12b, —CH2R12b, —C(O)R12b, —C(O)CH2R12b, —C(O)CH2NHR12b, —C(O)NRxR12b, —NRxC(O)CH2R12b, —NRxR12b, —NRxCH2R12b, —NHC(O)CH2NRxR12b, —NHC(O)CH2NRxCH2R12b, —NHCH2C(O)NHR12b, or —OR12b;
R12b is azetidinyl, cyclopropyl, diazabicyclo[2.2.1]heptanyl, dioxolanyl, dioxidotetrahydrothiopyranyl, dioxidothiomorpholinyl, imidazolyl, morpholinyl, octahydrocyclopenta[c]pyrrolyl, octahydropyrrolo[3,4-c]pyrrolyl, oxaazaspiro[3.3]heptanyl, oxetanyl, phenyl, piperazinyl, piperazinonyl, piperidinyl, pyridinyl, pyrrolidinyl, quinuclidinyl, tetrahydrofuranyl, tetrahydropyranyl, or triazolyl, each substituted with zero to 4 substituents independently selected from F, —OH, —CH3, —CH(CH3)2, —CH2OH, —OCH3, —CH2CH2OCH3, —NRxRx, and —C(O)NH2;
each Rx is independently H or —CH3;
n is zero; and
p is zero, 1, 2, or 3.
|