| CPC A61K 31/44 (2013.01) [A61K 9/0014 (2013.01); A61P 17/04 (2018.01)] | 16 Claims |
|
1. A method for preventing or treating atopic dermatitis, comprising a step of administering a compound represented by the following Formula 1 of an effective amount to a subject
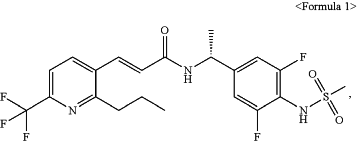 wherein the atopic dermatitis is mild or moderate (an Investigator's Global Assessment (IGA) score of 2 to 3), and
wherein the composition improves one or more parameters selected from the following parameters (a) to (c) associated with atopic dermatitis:
(a) improving an IGA grade of 2 for a patient (with mild symptoms) to grade 1 (almost clear symptoms) or 0 (clear symptoms);
(b) improving an IGA grade of 3 for a patient (with moderate symptoms) to grade 1 (almost clear symptoms) or 0 (clear symptoms); and
(c) improving an EASI score and reducing the EASI score by at least 40% or more.
|