| CPC A61K 31/428 (2013.01) [A61K 9/48 (2013.01); A61P 25/28 (2018.01)] | 7 Claims |
|
1. A method of treating Alzheimer's Disease of mild to moderate severity in a patient in need thereof, the method comprising administering to the patient a riluzole prodrug having the following formula:
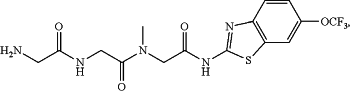 wherein the riluzole prodrug is administered to the patient at a dosage of 280 mg once per day or a dosage of 140 mg twice per day.
|