| CPC A61B 17/00234 (2013.01) [A61B 17/0482 (2013.01); A61B 17/06109 (2013.01); A61B 2017/00022 (2013.01); A61B 2017/00274 (2013.01); A61B 2017/00792 (2013.01); A61B 2017/00796 (2013.01); A61B 2017/00805 (2013.01); A61B 17/0218 (2013.01); A61B 17/0401 (2013.01); A61B 2017/0404 (2013.01); A61B 2017/0409 (2013.01); A61B 2017/0417 (2013.01); A61B 2017/0419 (2013.01); A61B 2017/0451 (2013.01); A61B 2017/0454 (2013.01); A61B 2017/0456 (2013.01); A61B 2017/0458 (2013.01); A61B 2017/0462 (2013.01); A61B 2017/0464 (2013.01); A61B 17/0467 (2013.01); A61B 17/0469 (2013.01); A61B 17/0487 (2013.01); A61B 2017/0488 (2013.01); A61B 2017/06052 (2013.01); A61B 2017/06176 (2013.01); A61B 17/3468 (2013.01); A61B 17/3478 (2013.01); A61B 17/42 (2013.01); A61B 2018/00547 (2013.01); A61F 2002/041 (2013.01); A61F 2/82 (2013.01)] | 16 Claims |
|
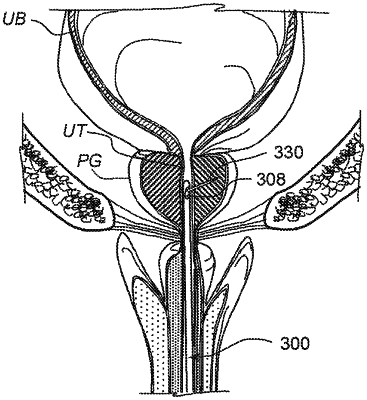
1. A method for treating benign prostatic hyperplasia, comprising:
inserting an interventional device comprising a tubular elongate device coupled with a hollow needle defining a sharp distal tip longitudinally through a urethra of a subject until a distal portion of the tubular elongate device reaches a position within the urethra distal to a prostate gland;
positioning the distal portion of the tubular elongate device at a first target region and advancing the hollow needle from an opening in the distal portion of the tubular elongate device into a lateral lobe of the prostate gland at the first target region;
injecting a therapeutic agent from the distal portion of the needle into the lateral lobe at the first target region;
repositioning the distal portion of the tubular elongate device at a second target region and advancing the hollow needle from the opening in the distal portion of the tubular elongate device into the lateral lobe at the second target region;
injecting more of the therapeutic agent from the distal portion of the needle into the lateral lobe at the second target region, wherein the therapeutic agent causes the lateral lobe to decrease in size; and
viewing a position of the interventional device using an endoscopic device advanced to the prostatic urethra.
|