| CPC C07C 255/50 (2013.01) [A61K 9/0053 (2013.01); A61P 3/00 (2018.01); A61P 33/00 (2018.01); C07C 217/18 (2013.01); C07C 217/48 (2013.01)] | 13 Claims |
|
1. A compound having formula (I):
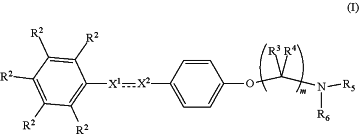 Including enantiomers, diastereomers, hydrates, solvates, pharmaceutically acceptable salts, prodrugs and complexes thereof, wherein:
the compound having the formula (I) is an N,N-dialkylamino phenyl ether compound,
the bond between X1 and X2 is selected from a double bond and a single bond,
when the bond between X1 and X2 is a single bond
X1 is CHR1
X2 is selected from CH2, CHR1, and CO;
X1 and X2 are not both CH2, and X1 and X2 are not both CHR1;
when the bond between X1 and X2 is a double bond
X1 is selected from CH and CR1
X2 is selected from CH and CR1
X1 and X2 are not both CH, and X1 and X2 are not both CR1;
R1 is selected from the group consisting of C1-10 linear alkyl, C3-10 branched alkyl, C3-10 cycloalkyl, C1-10 linear alkenyl, C3-10 branched alkenyl, C1-10 linear alkynyl, and C3-10 branched alkynyl;
R2 is at each occurrence independently selected from the group consisting of H, OH, CN, NO2, C1-10 alkoxy, C3-10 branched alkoxy, C1-10 haloalkoxy, C3-10 branched haloalkoxy, NR7R8, C(O)OR9, C1-10 thioalkyl, C3-10 branched thioalkyl, C1-10 halothioalkyl, —S(O)C1-10 alkyl, —S(O)C3-10 branched alkyl, —S(O)C1-10 haloalkyl, —S(O)C3-10 branched haloalkyl, —SO2C1-10 alkyl, —SO2C3-10 branched alkyl, —SO2C1-10 haloalkyl, —SO2C3-10 branched haloalkyl, SO2NR10R11, —NR10SO2R12, C(O)—NR10R11, wherein at least one instance of R2 is not H;
R3 is selected from the group consisting of hydrogen, C1-6 linear alkyl, C3-7 branched alkyl;
R4 is selected from the group consisting of hydrogen, C1-6 linear alkyl, C3-7 branched alkyl;
m is 2, 3, 4, 5, 6, 7, 8, 9, or 10;
R5 is selected from the group consisting of methyl, C3-6 linear alkyl, and C3-7 branched alkyl;
R6 is selected from the group consisting of methyl, C3-6 linear alkyl, and C3-7 branched alkyl;
In some embodiments, R5 and R6 are taken together with the atoms to which they are bound to form a ring containing 4 to 7 members, optionally containing a member selected from the group consisting of O, S, and NR13;
R7 is at each occurrence independently selected from the group consisting of hydrogen, C1-6 linear alkyl, C3-7 branched alkyl;
R8 is at each occurrence independently selected from the group consisting of hydrogen, C1-6 linear alkyl, C3-7 branched alkyl;
R9 is at each occurrence independently selected from the group consisting of hydrogen, C1-6 linear alkyl, C3-7 branched alkyl;
R10 is at each occurrence independently selected from the group consisting of hydrogen, C1-6 linear alkyl, C3-7 branched alkyl;
R11 is at each occurrence independently selected from the group consisting of hydrogen, C1-6 linear alkyl, C3-7 branched alkyl;
R12 is at each occurrence independently selected from the group consisting of hydrogen, C1-6 linear alkyl, C3-7 branched alkyl;
R13 is at each occurrence independently selected from the group consisting of hydrogen, C1-6 linear alkyl, C3-7 branched alkyl; and
the pharmaceutically acceptable salts are formed using bases or acids selected from the group consisting of acetic, propionic, lactic, benzenesulfonic, benzoic, camphorsulfonic, citric, tartaric, succinic, dichloroacetic, ethenesulfonic, formic, fumaric, gluconic, glutamic, hippuric, hydrobromic, isethionic, maleic, malic, malonic, mandelic, methanesulfonic, mucic, napthalenesulfonic, nitric, oxalic, pamoic, pantothenic, phosphoric, phthalic, succinic, sulfuric, toluenesulfonic, and any combination thereof;
wherein the following compounds are specifically excluded from the scope of the markush:
1-[4-[3-(diethylamino)propoxy]phenyl]-2-phenyl-1-Propanone;
1-[4-[2-(diethylamino)ethoxy]phenyl]-2-phenyl-1-pentanone;
2-phenyl-1-[4-[2-(1-piperidinyl)ethoxy]phenyl]-1-Butanone;
1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-phenyl-1-Butanone;
2-(4-aminophenyl)-1-[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]-1-Butanone;
2-(4-nitrophenyl)-1-[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]-1-Butanone;
1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-[4-(1-methylethyl)phenyl]-1-Butanone;
4′-(2-diethylaminoethoxy)-2-phenylButyrophenone;
2-(4-bromophenyl)-1-[4-[2-(dimethylamino)ethoxy]phenyl]-1-Butanone;
1-[3,5-dibromo-4-[2-(dimethylamino)ethoxy]phenyl]-2-phenyl-1-Butanone;
2-(4-bromophenyl)-1-[4-[2-(dimethylamino)ethoxy]phenyl]-1-Butanone;
1-[4-[[6-(dimethylamino)hexyl]oxy]phenyl]-2-phenyl-1-Butanone;
1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-(4-ethylphenyl)-1-Butanone;
1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-(4-fluorophenyl)-1-Butanone;
1-[4-[3-(diethylamino)propoxy]phenyl]-2-phenyl-1-Butanone;
1-[4-[[5-(dimethylamino)pentyl]oxy]phenyl]-2-phenyl-1-Butanone;
2-phenyl-1-[4-[2-(1-pyrrolidinyl)ethoxy]phenyl]-1-Butanone;
1-[4-[4-(dimethylamino)butoxy]phenyl]-2-phenyl-1-Butanone;
1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-(4-methylphenyl)-1-Butanone;
1-[4-[2-(diethylamino)ethoxy]phenyl]-3-methyl-2-phenyl-1-Butanone;
1-[4-[2-(diethylamino)ethoxy]phenyl]-2-phenyl-1-Butanone;
1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-phenyl-1-Butanone;
4′-(2-morpholinoethoxy)-2-phenyl-Butyrophenone;
1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-phenyl-1-Propanone;
2-cyclohexyl-1-[3,5-dimethyl-4-[2-(4-morpholinyl)ethoxy]phenyl]-2-phenyl-Ethanone;
N,N-dimethyl-2-[4-[(1Z)-2-phenyl-1-buten-1-yl]phenoxy]-Ethanamine;
N,N-diethyl-2-[4-[2-(4-methoxyphenyl)-1-propen-1-yl]phenoxy]-Ethanamine;
4-[(1E)-2-[4-[2-(diethylamino)ethoxy]phenyl]-1-methylethenyl]-Phenol;
4-[2-[4-[2-(diethylamino)ethoxy]phenyl]-1-methylethenyl]-Phenol;
2-[p-(p-methoxy-b-methylstyryl)phenoxy]-Triethylamine;
N,N-diethyl-3-[4-(1-methyl-2-phenylethenyl)phenoxy]-1-Propanamine;
N,N-diethyl-2-[4-[1-(phenylmethylene)propyl]phenoxy]-Ethanamine;
N,N-diethyl-2-[4-(1-methyl-2-phenylethenyl)phenoxy]-Ethanamine;
2-[p-(p-methoxy-a-methylstyryl)phenoxy]-Triethylamine;
4′-[2-(diethylamino)ethoxy]-a′-methyl-4-Stilbenol;
(E)-1-[2-[4-(1-cyclopentyl-2-phenylethenyl)phenoxy]ethyl]-Pyrrolidine;
(Z)-1-[2-[4-(1-cyclopentyl-2-phenylethenyl)phenoxy]ethyl]-Pyrrolidine;
2-[4-[1-cyclohexyl-2-(4-methoxyphenyl)ethenyl]phenoxy]-N,N-diethyl-Ethanamine;
N,N-diethyl-2-[4-[2-(4-methoxyphenyl)propyl]phenoxy]-Ethanamine;
4-[2-[4-[2-(diethylamino)ethoxy]phenyl]-1-methylethyl]-Phenol;
N,N-diethyl-3-[4-(1-methyl-2-phenylethyl)phenoxy]-1-Propanamine;
N,N-diethyl-2-[4-(1-methyl-2-phenylethyl)phenoxy]-Ethanamine;
N,N-diethyl-2-[4-[1-[(4-methoxyphenyl)methyl]propyl]phenoxy]-Ethanamine;
N,N-diethyl-2-[4-[2-(4-methoxyphenyl)-1-methylethyl]phenoxy]-Ethanamine;
4-[2-[4-[2-(diethylamino)ethoxy]phenyl]propyl]-Phenol;
and 2-[4-[1-cyclohexyl-2-(4-methoxyphenyl)ethyl]phenoxy]-N,N-diethyl-Ethanamine.
|