| CPC C01F 11/22 (2013.01) [B01J 10/00 (2013.01); C01B 33/186 (2013.01); C01C 1/026 (2013.01); C01C 1/26 (2013.01); C01P 2004/61 (2013.01); C01P 2006/12 (2013.01); C01P 2006/80 (2013.01)] | 10 Claims |
|
1. A process for preparing synthetic calcium fluoride (CaF2) from fluosilicic acid, comprising the following steps:
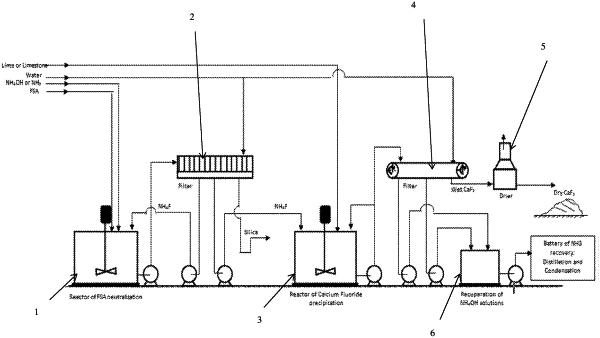
(a) directly reacting fluosilicic acid (H2SiF6) with ammonium hydroxide or ammonia in a first reactor so as to obtain a first slurry; filtering said first slurry so as to obtain a filtrate containing a solution of ammonium fluoride;
(b) precipitating ammonium fluoride the solution of ammonium fluoride obtained as a filtrate in step (a) with calcium carbonate (CaCO3) as a dry form or as a suspension at a concentration ranging from 10 to 80% by weight in a second reactor so as to produce a second slurry containing a precipitate of calcium fluoride and ammonium carbonate; producing a first ammonium carbonate solution by filtering said second slurry so as to obtain a filter cake containing calcium fluoride and a filtrate containing the solution of ammonium carbonate; producing a second ammonium carbonate solution by washing and drying said filter cake so as to obtain calcium fluoride and a filter cake washing containing the second solution of ammonium carbonate; wherein a portion of the second slurry ranging from 10 to 70% is recycled to the second reactor so as to enhance calcium fluoride crystallization in the second reactor;
(c) evolving the major part of ammonia from the second reactor in step (b) because of partial decomposition of ammonium carbonate under the second reactor conditions and then scrubbing and returning said ammonia to the first reactor; gathering and treating by distillation and condensation the first and second ammonium carbonate solutions to recover liquid ammonia; and recycling the recovered liquid ammonia to the first reactor,
wherein the calcium fluoride obtained at step (b) presents a purity greater than or equal to 90% wt CaF2.
|