| CPC A61K 38/4886 (2013.01) [A61K 9/0019 (2013.01); A61K 9/0021 (2013.01); A61K 9/0034 (2013.01); A61P 15/00 (2018.01); A61B 5/4824 (2013.01); C12Y 304/24003 (2013.01)] | 12 Claims |
|
1. A method for reducing the size of a uterine fibroid in a patient, the method comprising:
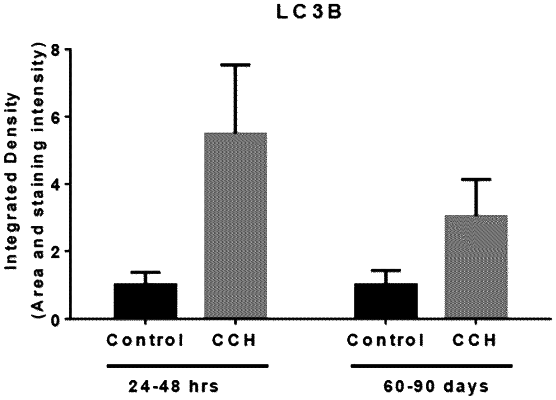
administering into the uterine fibroid a composition comprising Clostridium histolyticum collagenase I and collagenase II in a 1:1 mass ratio to thereby reduce the size of the uterine fibroid, wherein there is an at least two fold increase in LC3B expression within the uterine fibroid as measured at 60 days following the administration.
|