| CPC A61K 31/7028 (2013.01) [A61K 47/544 (2017.08); A61P 21/00 (2018.01); C12N 15/113 (2013.01)] | 12 Claims |
|
1. A composition comprising:
a. an oligomeric compound comprising one or more tricyclo-deoxyribonucleic acid (tc-DNA) nucleosides, wherein the oligomeric compound comprises from 5 to 40 monomer subunits; and
b. a lipid moiety of formula C3-32alkyl-C(O)—*,
wherein the lipid moiety is covalently linked to the oligomeric compound at the 5′ terminus or at the 3′ terminus of the oligomeric compound either directly or via a spacer;
wherein at least one tc-DNA nucleoside is of Formula (1):
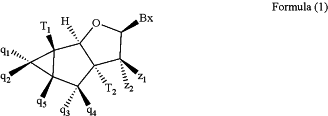 wherein in Formula (1):
Bx is a nucleobase;
one of T1 and T2 is an internucleosidic linkage group, and the other of T1 and T2 is OR1, OR2, a 5′ terminal group, a 3′ terminal group or a internucleosidic linkage group, wherein R1 is H or a hydroxyl protecting group, and R2 is a phosphorus moiety;
q1, q2, q3, q4 and q5 are each independently selected from the group consisting of hydrogen (H), halogen, C1-6alkyl, C2-6alkenyl, C2-6alkynyl, substituted C1-6alkyl, substituted C2-6alkenyl, substituted C2-6alkynyl, and —(CH2)n—C(O)—R6′, wherein n is 0 to 6 and wherein R6′ is selected from the group consisting of OH, NH2, O—C1-32alkyl and NH—C1-32alkyl;
z1 and z2 are each independently selected from the group consisting of H, halogen, C1-6alkyl, C1-6alkoxyl, O—C2-6alkenyl, O—C2-6alkynyl, substituted C1-6alkyl, substituted C1-6alkoxy, substituted O—C2-6alkenyl, and substituted O—C2-6alkynyl;
or a pharmaceutically acceptable salt thereof, and
wherein the oligomeric compound is at least 50% complementary to a splice donor and/or acceptor site within a dystrophin pre-mRNA sequence.
|