| CPC A61K 31/5025 (2013.01) [A61K 47/10 (2013.01); A61K 47/32 (2013.01); A61K 47/38 (2013.01)] | 16 Claims |
|
1. An amorphous solid dispersion comprising a compound of Formula (I) or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable polymer;
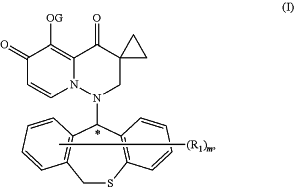 wherein
G is hydrogen or —C(R2R2′) —O—C═O)—R3, in which each of R2 and R2′independently is hydrogen or C1-4 alkyl; R3 is C1-4 alkyl;
R1 is halogen, deuterium, or C1-4 alkyl;
m is an integer of 1 to 9; and
the star (*) indicates a chiral center:,
wherein the pharmaceutically acceptable polymer is polyvinylpyrrolidone/vinyl acetate copolymer, polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer, methacrylic acid and methyl methacrylate copolymer, hydroxypropyl cellulose (HPC), hydroxypropyl methyl cellulose acetate succinate (HPMCAS), hydroxypropyl methyl cellulose phthalate (HPMCP), Polyethylene glycol (PEG), or Kollidon SR; and
wherein the weight ratio of the compound of Formula (I) or a pharmaceutically acceptable salt thereof to the pharmaceutically acceptable polymer is in a range between about 1:1 to about 1:5.
|