| CPC A61B 5/085 (2013.01) [A61B 5/4815 (2013.01); A61B 5/02055 (2013.01); A61M 16/0051 (2013.01); A61M 16/0069 (2014.02); A61M 2016/0027 (2013.01); A61M 2016/0039 (2013.01); A61M 2202/0208 (2013.01); A61M 2205/3553 (2013.01); A61M 2205/3561 (2013.01); A61M 2205/3584 (2013.01); A61M 2205/3592 (2013.01); A61M 2230/04 (2013.01); A61M 2230/10 (2013.01); A61M 2230/14 (2013.01); A61M 2230/202 (2013.01); A61M 2230/205 (2013.01); A61M 2230/60 (2013.01)] | 20 Claims |
|
1. A positive airway pressure (PAP) sleep disorder treatment system comprising:
a non-transitory computer readable medium comprising a first software, the first software adapted to be downloaded to a cell phone and executable by a first processor on the cell phone to display to a subject for viewing on the cell phone an index of treatment efficacy of the subject using a PAP device, the index based in whole or in part on a data of severity of sleep disorder symptoms of the subject and/or a data of usage of the PAP device by the subject, the cell phone comprising at least one first radio frequency wireless transceiver, the first processor, the first software when downloaded, and a display;
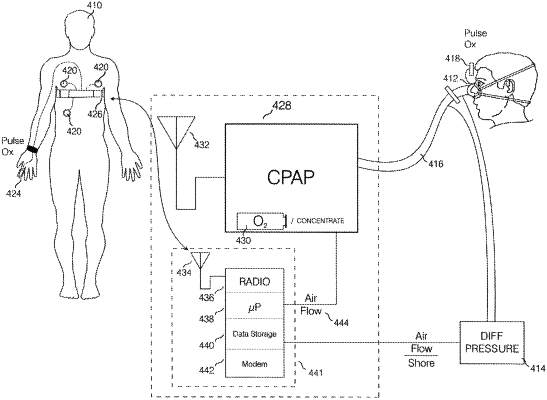
the PAP device with an enclosure further comprising:
a blower having an air output within the enclosure,
an airflow sensor being any type of sensor internal to the enclosure of the PAP device adapted for measuring or deriving the respiratory airflow of a subject while using the PAP device and outputting airflow sensor data,
a second processor within the enclosure adapted for receiving the airflow sensor data and for calculating:
i) the data of a severity of the sleep disorder symptoms of the subject,
ii) the data of usage of the PAP device by the subject, and/or
iii) the index based in whole or in part on either i) or ii) or both i) and ii), a mask or a nasal cannula connected to the enclosure, and
at least one second radio frequency wireless transceiver within the enclosure, the at least one second radio frequency wireless transceiver adapted for transmitting i) and ii), and/or iii); and
a remote internet site, the remote internet site adapted for receiving i) and ii), or i), ii), and iii), related to the subject's treatment and the treatment's efficacy in whole or part, generated by the PAP device and transmitted via the at least one second radio frequency wireless transceiver.
|