| CPC A23L 27/86 (2016.08) [C07D 317/66 (2013.01); C07D 401/04 (2013.01); C07D 405/12 (2013.01); C07D 405/14 (2013.01); C07D 407/12 (2013.01); C07D 409/14 (2013.01); C07D 413/12 (2013.01); C07D 413/14 (2013.01)] | 12 Claims |
|
1. A compound of formula (I)
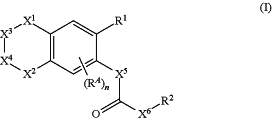 or a salt thereof, wherein:
X1 and X2 are each an oxygen atom;
X3 is a direct bond or >CH2;
X4 is >CH2;
RA is a halogen atom, —CN, nitro, —OH, —NH2, —C(O)H, —O—C(O)H, —C(O)—OH, —NH—C(O)H, —C(O)—NH2, —O—(C1-6alkyl), —NH—(C1-6alkyl), —N(C1-6 alkyl)2, —C(O)—(C1-6alkyl), —O—C(O)—(C1-6alkyl), —NH—C(O)—(C1-6alkyl), —N(C1-6alkyl)-C(O)—(C1-6alkyl), —C(O)—O—(C1-6alkyl), —C(O)—NH—(C1-6alkyl), —C(O)—N(C1-6alkyl)2, —S(O)2—(C1-6alkyl), —O—S(O)2—(C1-6alkyl), —NH—S(O)2—(C1-6), —N(C1-6alkyl)-S(O)2—(C1-6alkyl), —S(O)2—(C1-6alkyl), —S(O)2—NH—(C1-6alkyl), —S(O)2—N(C1-6alkyl)2, C3-10cycloalkyl, C2-14 heterocyclyl, C6-14 aryl, C2-14 heteroaryl, C1-6 alkyl, C2-6alkenyl, C1-6haloalkyl, C2-6haloalkenyl, C1-6haloalkoxy, C2-6haloalkenyloxy, or (C1-6alkoxy)-C1-6alkyl;
R1 is —C(O)—R1A;
R1A is C1-6 alkyl, which is optionally substituted one or more times by substituents selected independently from R1Y;
R1Y is a halogen atom, oxo, —CN, nitro, —OH, —NH2, —C(O)H, —O—C(O)H, —C(O)—OH, —NH—C(O)H, —C(O)—NH2, —O—(C1-6alkyl), —NH—(C1-6 alkyl), —N(C1-6alkyl)2, —C(O)—(C1-6alkyl), —O—C(O)—(C1-6alkyl), —NH—C(O)—(C1-6alkyl), —C(O)—O—(C1-6alkyl), —C(O)—NH—(C1-6alkyl), —C(O)—N(C1-6alkyl)2, —S(O)2—(C1-6alkyl), —O—S(O)2—(C1-6alkyl), —NH—S(O)2—(C1-6alkyl), —S(O)2—O—(C1-6alkyl), —S(O)2—NH—(C1-6alkyl), —S(O)2—N(C1-6alkyl)2, C3-10cycloalkyl, C2-14 heterocyclyl, C6-14 aryl, C2-14 heteroaryl, C1-6alkyl, C2-6alkenyl, C1-6haloalkyl, C2-6haloalkenyl, C1-6haloalkoxy, C2-6haloalkenyloxy, or (C1-6alkoxy)-C1-6alkyl;
R2 is
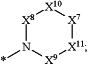 X5 is >NR2G;
X6 is C1-6 alkylene, which is optionally substituted one or more times by substituents selected independently from R1Y;
X7 is >N—R2L;
X8, X9, X10, and X11 are each independently >C(R2M)(R2N);
R2M and R2N are each independently a hydrogen atom, a halogen atom, —CN, nitro, —OH, —NH2, —C(O)H, —O—C(O)H, —C(O)—OH, —NH—C(O)H, —C(O)—NH2, —O—(C1-6alkyl), —NH—(C1-6alkyl), —N(C1-6alkyl)2, —C(O)—(C1-6alkyl), —O—C(O)—(C1-6alkyl), —NH—C(O)—(C1-6alkyl), —NH—C(O)—O—(C1-6alkyl), —C(O)—O—(C1-6alkyl), —C(O)—NH—(C1-6alkyl), —C(O)—N(C1-6alkyl)2, —S(O)2—(C1-6alkyl), —O—S(O)2—(C1-6alkyl), —NH—S(O)2—(C1-6alkyl), —S(O)2—O—(C1-6alkyl), —S(O)2—NH—(C1-6alkyl), —S(O)2—N(C1-6alkyl)2, C3-10cycloalkyl, C2-14 heterocyclyl, C6-14 aryl, C2-14 heteroaryl, C1-6alkyl, C2-6alkenyl, C1-6haloalkyl, C2-6haloalkenyl, C1-6haloalkoxy, C2-6 haloalkenyloxy, or (C1-6alkoxy)-C1-6alkyl; or R2M and R2N optionally combine to form an oxo group; or any two of R2M and R2N attached to adjacent carbon atoms of the piperazine ring defined by R2 optionally combine to form a fused ring selected from the group consisting of phenyl, C2-5 heteroaryl, C4-8 cycloalkyl, and C2-5 heterocyclyl, wherein each of the fused rings is optionally substituted one or more times by substituents selected independently from R1Z;
R1Z is a halogen atom, oxo, —CN, nitro, —OH, —NH2, —C(O)H, —O—C(O)H, —C(O)—OH, —NH—C(O)H, —C(O)—NH2, —O—(C1-6alkyl), —NH—(C1-6alkyl), —N(C1-6 alkyl)2, —C(O)—(C1-6alkyl), —O—C(O)—(C1-6alkyl), —NH—C(O)—(C1-6alkyl), —C(O)—O—(C1-6alkyl), —C(O)—NH—(C1-6alkyl), —C(O)—N(C1-6alkyl)2, —S(O)2—(C1-6alkyl), —O—S(O)2—(C1-6alkyl), —NH—S(O)2—(C1-6alkyl), —S(O)2—O—(C1-6alkyl), —S(O)2—NH—(C1-6alkyl), —S(O)2—N(C1-6alkyl)2, C3-10cycloalkyl, C2-14 heterocyclyl, C6-14 aryl, C2-14 heteroaryl, C1-6alkyl, C2-6alkenyl, C1-6haloalkyl, C2-6haloalkenyl, C1-6haloalkoxy, C2-6haloalkenyloxy, (C1-6alkoxy)-C1-6alkyl, C1-6alkyl, or C2-6alkenyl;
R2G is a hydrogen atom, C1-6alkyl, C1-6haloalkyl, or (C1-6alkoxy)-C1-6alkyl;
R2L is —C(O)—R3 or —C(O)—X14—R4;
X14 is C1-8alkylene, which is optionally substituted by one or more times by substituents selected independently from R1Y;
R3 is a hydrogen atom, —OH, —NH2, —O—R3B, —NH—R3B, —N(R3B)(R3C), —O—C(O)—R3B, —NH—C(O)—R3B, —N(R3C)—C(O)—R3B, C3-10 carbocyclyl, C2-14 heterocyclyl, C6-14 aryl, or C2-14 heteroaryl, wherein the carbocyclyl, heterocyclyl, aryl, and heteroaryl are each optionally substituted one or more times by substituents selected independently from R3A, wherein any two R3A attached to adjacent carbon atoms of the carbocyclyl, heterocyclyl, aryl, and heteroaryl groups to which they are attached optionally combine to form a fused ring selected from the group consisting of phenyl, C2-5 heteroaryl, C4-8cycloalkyl, and C2-5 heterocyclyl, wherein each of the fused rings is optionally substituted one or more times by substituents selected independently from R1Z, and wherein, when R3B and R3C are attached to the same nitrogen atom, R3B and R3C optionally combine with the nitrogen atom to which they are attached to form a nitrogen-containing C2-6 heterocyclic ring, which is optionally substituted by C1-4 alkyl;
R3A is a halogen atom, —CN, nitro, oxo, —OH, —NH2, —C(O)H, —O—C(O)H, —C(O)—OH, —NH—C(O)H, —C(O)—NH2, —O—R3D, —NH—R3D, —N(R3D)(R3E), —C(O)—R3D, —O—C(O)—R3D, —NH—C(O)—R3D, —C(O)—O—R3D, —C(O)—NH—R3D, —C(O)—N(R3D)(R3E), —S(O)2—R3D, —O—S(O)2—R3D, —NH—S(O)2—R3D, —S(O)2—O—R3D, —S(O)2—NH—R3D, —S(O)2—N(R3D)(R3E), C1-8alkyl, C2-8alkenyl, C3-10 carbocyclyl, C2-14 heterocyclyl, C6-14 aryl, or C2-14 heteroaryl, wherein the alkyl and alkenyl are each optionally substituted one or more times by substituents selected independently from R3Y, and wherein the carbocyclyl, heterocyclyl, aryl, and heteroaryl are each optionally substituted one or more times by substituents selected independently from R3Z;
R3B, R3C, R3D, and R3E are each independently C1-8alkyl, C2-8 alkenyl, C3-10 carbocyclyl, C2-14 heterocyclyl, C6-14 aryl, or C2-14 heteroaryl, wherein the alkyl and alkenyl are each optionally substituted one or more times by substituents selected independently from R3Y, and wherein the carbocyclyl, heterocyclyl, aryl, and heteroaryl are each optionally substituted one or more times by substituents selected independently from R3Z;
R3Y is a halogen atom, —CN, nitro, oxo, —OH, —NH2, —C(O)H, —O—C(O)H, —C(O)—OH, —NH—C(O)H, —C(O)—NH2, —O—(C1-6alkyl), —NH—(C1-6alkyl), —N(C1-6alkyl)2, —C(O)—(C1-6alkyl), —O—C(O)—(C1-6alkyl), —NH—C(O)—(C1-6alkyl), —N(C1-6alkyl)-C(O)—(C1-6alkyl), —C(O)—O—(C1-6alkyl), —C(O)—NH—(C1-6alkyl), —C(O)—N(C1-6alkyl)2, —S(O)2—(C1-6alkyl), —O—S(O)2—(C1-6alkyl), —NH—S(O)2—(C1-6alkyl), —N(C1-6alkyl)-S(O)2—(C1-6alkyl), —S(O)2—O—(C1-6alkyl), —S(O)2—NH—(C1-6alkyl), —S(O)2—N(C1-6alkyl)2, —O—(C1-6alkylene)-O—(C1-6alkyl), C3-10cycloalkyl, C2-14 heterocyclyl, C6-14 aryl, or C2-14 heteroaryl, wherein the cycloalkyl, heterocyclyl, aryl, and heteroaryl groups are each optionally substituted one or more times by substituents selected independently from R1Z;
R3Z is a halogen atom, —CN, nitro, oxo, —OH, —NH2, —C(O)H, —O—C(O)H, —C(O)—OH, —NH—C(O)H, —C(O)—NH2, —O—(C1-6alkyl), —NH—(C1-6alkyl), —N(C1-6alkyl)2, —C(O)—(C1-6alkyl), —O—C(O)—(C1-6alkyl), —NH—C(O)—(C1-6alkyl), —N(C1-6alkyl)-C(O)—(C1-6alkyl), —C(O)—O—(C1-6alkyl), —C(O)—NH—(C1-6alkyl), —C(O)—N(C1-6alkyl)2, —S(O)2—(C1-6alkyl), —O—S(O)2—(C1-6alkyl), —NH—S(O)2—(C1-6alkyl), —N(C1-6alkyl)-S(O)2—(C1-6alkyl), —S(O)2—O—(C1-6alkyl), —S(O)2—NH—(C1-6alkyl), —S(O)2—N(C1-6alkyl)2, C1-6alkyl, C2-6alkenyl, C1-6haloalkyl, C2-6haloalkenyl, C1-6haloalkoxy, C2-6haloalkenyloxy, (C1-6alkoxy)-C1-6alkyl, C3-10cycloalkyl, C2-14 heterocyclyl, C6-14 aryl, or C2-14 heteroaryl, wherein the cycloalkyl, heterocyclyl, aryl, and heteroaryl groups are each optionally substituted one or more times by substituents selected independently from R1Z;
R4 is a hydrogen atom, a halogen atom, —CN, nitro, oxo, —OH, —NH2, —O—R4B, —NH—R4B, —N(R4B)(R4C), C(O)—R4B, —O—C(O)—R4B, —NH—C(O)—R4B, —N(R4C)—C(O)—R4B, —C(O)—O—R4B, —C(O)—NH—R4B, —C(O)N(R4B)(R4C), —S(O)2—R4D, —O—S(O)2—R4D, —NH—S(O)2—R4D, —S(O)2—O—R4D, —S(O)2—NH—4D, —S(O)2—N(R4D)(R4E), C3-10 carbocyclyl, C2-14 heterocyclyl, C6-14 aryl, or C2-14 heteroaryl, wherein the carbocyclyl, heterocyclyl, aryl, and heteroaryl are each optionally substituted one or more times by substituents selected independently from R4A, wherein any two R4A attached to adjacent carbon atoms of the carbocyclyl, heterocyclyl, aryl, and heteroaryl groups to which they are attached optionally combine to form a fused ring selected from the group consisting of phenyl, C2-5 heteroaryl, C4-8cycloalkyl, or C2-5 heterocyclyl, wherein each of the fused rings is optionally substituted one or more times by substituents selected independently from R1Z, and wherein, when R4B and R4C are attached to the same nitrogen atom, R4B and R4C optionally combine with the nitrogen atom to which they are attached to form a nitrogen-containing C2-6 heterocyclic ring, which is optionally substituted by C1-4 alkyl;
R4A is a halogen atom, —CN, nitro, oxo, —OH, —NH2, —C(O)H, —O—C(O)H, —C(O)—OH, —NH—C(O)H, —C(O)—NH2, —O—R4D, —NH—R4D, —N(R4D)(R4E), —C(O)—R4D, —O—C(O)—R4D, —NH—C(O)—R4D, —N(R4E)—C(O)—R4D, —C(O)—O—R4D, —C(O)—NH—R4D, —C(O)—N(R4D)(R4E), C1-8alkyl, C2-8alkenyl, C3-10 carbocyclyl, C2-14 heterocyclyl, C6-14 aryl, or C2-14 heteroaryl, wherein the alkyl and alkenyl are each optionally substituted one or more times by substituents selected independently from R4Y, and wherein the carbocyclyl, heterocyclyl, aryl, and heteroaryl are each optionally substituted one or more times by substituents selected independently from R4Z;
R4B, R4C, R4D, and R4E are each independently C1-8 alkyl, C2-8 alkenyl, C3-10 carbocyclyl, C2-14 heterocyclyl, C6-14 aryl, or C2-14 heteroaryl, wherein the alkyl and alkenyl are each optionally substituted one or more times by substituents selected independently from R″, and wherein the carbocyclyl, heterocyclyl, aryl, and heteroaryl are each optionally substituted one or more times by substituents selected independently from R4Z;
R4Y is a halogen atom, —CN, nitro, oxo, —OH, —NH2, —C(O)H, —O—C(O)H, —C(O)—OH, —NH—C(O)H, —C(O)—NH2, —O—(C1-6alkyl), —NH—(C1-6alkyl), —N(C1-6alkyl)2, —C(O)—(C1-6alkyl), —O—C(O)—(C1-6alkyl), —NH—C(O)—(C1-6alkyl), —N(C1-6alkyl)-C(O)—(C1-6alkyl), —C(O)—O—(C1-6alkyl), —C(O)—NH—(C1-6alkyl), —C(O)—N(C1-6alkyl)2, —S(O)2—(C1-6alkyl), —O—S(O)2—(C1-6alkyl), —NH—S(O)2—(C1-6alkyl), —N(C1-6alkyl)-S(O)2—(C1-6alkyl), —S(O)2—O—(C1-6alkyl), —S(O)2—NH—(C1-6alkyl), —S(O)2—N(C1-6alkyl)2, —O—(C1-6alkylene)-O—(C1-6alkyl), C3-10 cycloalkyl, C2-14 heterocyclyl, C6-14 aryl, or C2-14 heteroaryl, wherein the cycloalkyl, heterocyclyl, aryl, and heteroaryl groups are each optionally substituted one or more times by substituents selected independently from R1Z;
R4Z is a halogen atom, —CN, nitro, oxo, —OH, —NH2, —C(O)H, —O—C(O)H, —C(O)—OH, —NH—C(O)H, —C(O)—NH2, —O—(C1-6alkyl), —NH—(C1-6alkyl), —N(C1-6alkyl)2, —C(O)—(C1-6alkyl), —O—C(O)—(C1-6alkyl), —NH—C(O)—(C1-6alkyl), —N(C1-6alkyl)-C(O)—(C1-6alkyl), —C(O)—O—(C1-6alkyl), —C(O)—NH—(C1-6alkyl), —C(O)—N(C1-6alkyl)2, —S(O)2—(C1-6alkyl), —O—S(O)2—(C1-6alkyl), —NH—S(O)2—(C1-6alkyl), —N(C1-6alkyl)-S(O)2—(C1-6alkyl), —S(O)2—O—(C1-6alkyl), —S(O)2—NH—(C1-6 alkyl), —S(O)2—N(C1-6alkyl)2, (C1-6alkoxy)-C1-6 alkyl, C3-10cycloalkyl, C2-14 heterocyclyl, C6-14 aryl, C2-14 heteroaryl, C1-6alkyl, C2-6alkenyl, C1-6haloalkyl, C2-6haloalkenyl, C1-6haloalkoxy, or C2-6haloalkenyloxy, wherein the cycloalkyl, heterocyclyl, aryl, and heteroaryl groups are each optionally substituted one or more times by substituents selected independently from R1Z; and
n is 0, 1, or 2.
|