| CPC H01M 4/525 (2013.01) [H01M 4/0471 (2013.01); H01M 4/364 (2013.01); H01M 4/382 (2013.01); H01M 4/505 (2013.01); H01M 2004/021 (2013.01); H01M 2004/028 (2013.01)] | 15 Claims |
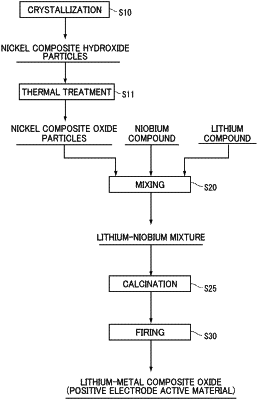
|
1. A positive electrode active material for non-aqueous electrolyte secondary battery comprising a lithium-nickel-manganese composite oxide formed of secondary particles with a plurality of aggregated primary particles, wherein
the positive electrode active material is represented by a general formula (1): LidNi1−a−b−cMnaMbNbcO2+α [where M is at least one element selected from the group consisting of Co, W, Mo, V, Mg, Ca, Al, Ti, Cr, Zr and Ta, 0.05≤a≤0.6, 0≤b≤0.6, 0.0003≤c≤0.03, 0.95≤d≤1.2, 0.33<(1−a−b−c), and 0≤α≤0.4],
at least a part of niobium is solid-dissolved inside the primary particles, and
an amount of lithium to be eluted into water when the positive electrode active material is immersed in water is 0.02% by mass or more and 0.10% by mass or less with respect to entire positive electrode active material as determined by a neutralization titration method.
|