| CPC G01N 33/54386 (2013.01) [B01L 3/5023 (2013.01); B01L 2400/0406 (2013.01); G01N 2333/99 (2013.01)] | 19 Claims |
|
1. A lateral flow device for the semi-quantitative detection of a cerebrospinal fluid (CSF) leak using a nasal drip or otologic sample, said device comprising:
a porous substrate;
a sample addition zone disposed on or in said porous substrate;
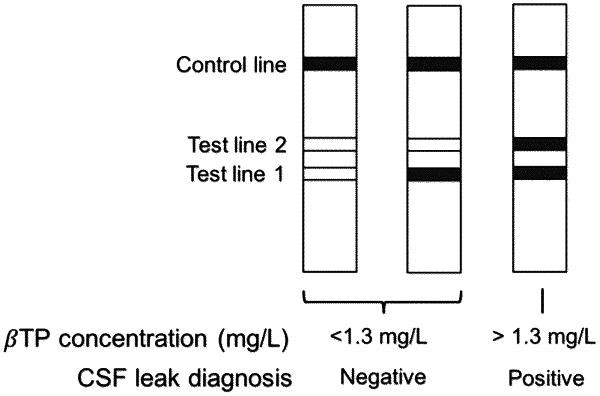
a detection zone disposed on or in said porous substrate where said detection zone comprises at least a first test line (T1) and a second test line (T2) each test line comprising binding moieties that bind a complex formed between beta-trace protein (βTP) and an indicator attached to a βTP binding molecule;
wherein said porous substrate defines a flow path through which a sample applied to the sample addition zone flows under capillary action away from said sample addition zone into said detection zone; and
wherein when a nasal drip sample diluted 300 fold with phosphate buffered saline is applied to said device:
said first test line (T1) and said second test line (T2) are configured so that either no test line signal or just a signal at the first test line (T1) is detectable when βTP concentration in a sample applied to said device is lower than the βTP level indicative of a CSF leak; and
said second test line (T2) is configured so that a signal is detectable at said second test line when βTP concentration in a sample applied to said device is greater than the βTP level indicative of a CSF leak.
|