| CPC C07D 487/10 (2013.01) [C07D 401/14 (2013.01); C07D 413/14 (2013.01); C07D 417/14 (2013.01); C07D 471/10 (2013.01); C07D 487/04 (2013.01); C07D 491/107 (2013.01); C07D 498/10 (2013.01)] | 37 Claims |
|
1. A compound of Formula (A)
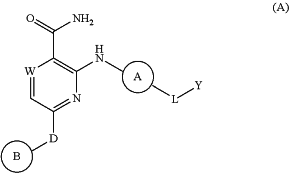 or a pharmaceutically acceptable salt thereof, wherein
W is N;
D is a bond;
Ring A is phenyl, optionally and independently substituted with up to 3 substituents selected from halo, —CN, —COOH, NH2, and optionally substituted C1-6 alkyl;
Ring B is a
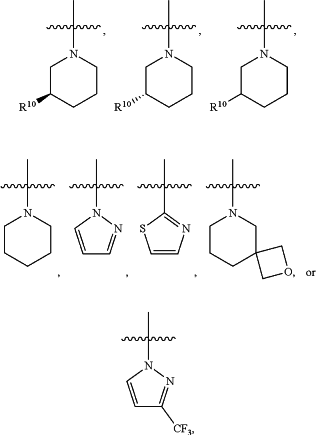 wherein R10 is
 L is —X1—X2—X3—X4—X5—;
X1 is a bond, —C(O)—, —C(O)—N(R)—, —N(R)—C(O)—, —(O—CH2—CH2)m—, —O(C6H4)—, —(O—CH2—CH2—CH2)m—, —C1-5 alkyl-, 7-12 membered spiro or fused bicyclic heterocycloalkyl having 1-3 heteroatoms independently selected from N, O, or S, or 4-6 membered monocyclic heterocycloalkyl having 1-2 heteroatoms independently selected from N, O, or S, wherein each of the monocyclic and bicyclic heterocycloalkyl of X1 is optionally substituted with —CH3;
X2 is a bond, —(O—CH2—CH2)n—, —(CH2—CH2—O)n—, —N(R)—C(O)—, —N(R)—, —C(O)—, —C(O)—N(R)—, —C1-5 alkyl-, 4-6 membered monocyclic cycloalkyl, or 4-6 membered monocyclic heterocycloalkyl having 1-2 heteroatoms independently selected from N, O, or S;
X3 is a bond, —C1-8 alkyl-,
 4-6 membered cycloalkyl, —N(R)—, —N(R)—C(O)—, —(O—CH2—CH2)p—, —(CH2—CH2—O)p—, 4-6 membered heterocycloalkyl having 1-2 heteroatoms independently selected from N, O, or S wherein the heterocycloalkyl is optionally substituted with —CH3;
X4 is a bond, —CH2—CH2—N(R)—, —N(R)—, —C1-4 alkyl-, —(O—CH2—CH2—CH2)m—, a 5-6 membered saturated, partially unsaturated, or fully unsaturated carbocycle, or a 5-6 membered saturated, partially unsaturated, or fully unsaturated heterocycle having 1-3 heteroatoms independently selected from N, O, or S;
X5 is a bond, —C1-4 alkyl-, —N(R)—, —O—, —C(O)—, or —C(O)—N(R)—;
each R is independently —H or —C1-3 alkyl; and
each of m, n, and p is independently an integer from 1 to 3; and
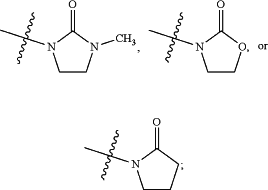
Y is
 |