| CPC C07B 31/00 (2013.01) [C07C 41/20 (2013.01); C07C 51/347 (2013.01); C07C 67/30 (2013.01); C07C 2523/04 (2013.01)] | 15 Claims |
|
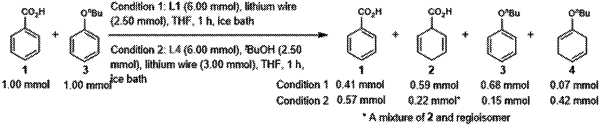
1. A method of reducing an aromatic ring or cyclic, allylic ether in a compound, comprising:
preparing a reaction mixture comprising a compound comprising an aromatic moiety or a cyclic, allylic ether moiety, an alkali metal, and either ethylenediamine, diethylenetriamine, thiethylenetetramine, or a combination thereof, in an ether solvent, wherein the ethylenediamine:compound molar ratio or the diethylenetriamine:compound molar ratio in the reaction mixture ranges from 2-20:1; and
reacting the reaction mixture at a temperature ranging from −20° C. to 30° C. for a time sufficient to reduce a double bond in the aromatic moiety to a single bond or to reduce the cyclic, allylic ether moiety.
|