| CPC A61K 31/716 (2013.01) [A61K 47/61 (2017.08); C07K 16/2863 (2013.01); C07K 16/32 (2013.01); C07K 2317/24 (2013.01); C07K 2317/73 (2013.01); C07K 2317/90 (2013.01)] | 17 Claims |
|
1. A composition comprising conjugates according to Formula II:
 wherein:
each Lys is a different lysine residue present in the Therapeutic Antibody;
b represents the average number of individual β-1,6-glucan oligomers that are conjugated to each Therapeutic Antibody molecule in the composition and is between 2 and 4; and
each
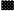 is a β-1,6-glucan oligomer of Formula Ia: is a β-1,6-glucan oligomer of Formula Ia: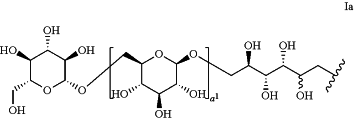 wherein:
a1 is 4; and
“
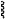 ” represents a point of attachment between the β-1,6-glucan oligomer and one lysine residue of the Therapeutic Antibody. ” represents a point of attachment between the β-1,6-glucan oligomer and one lysine residue of the Therapeutic Antibody. |