| CPC H10K 85/657 (2023.02) [C07D 403/04 (2013.01); C07D 403/14 (2013.01); C07D 471/06 (2013.01); C07D 471/16 (2013.01); C07D 491/06 (2013.01); C07D 491/16 (2013.01); C07D 495/06 (2013.01); C07D 495/16 (2013.01); C07D 495/22 (2013.01); C07D 519/00 (2013.01); C09K 11/06 (2013.01); H10K 85/6572 (2023.02); H10K 85/6574 (2023.02); C09K 2211/1018 (2013.01); H10K 50/11 (2023.02); H10K 2101/10 (2023.02)] | 13 Claims |
|
1. Compound comprising at least one structure of the (IIIa), (IIIb), (IIIc) or (IIId)
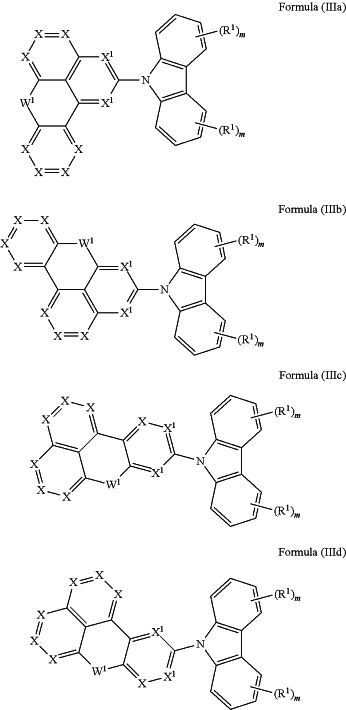 where in Formula (IIIa), (IIIb), (IIIc), and (IIId) W1 is SO, SO2, Si(R1)2 or C═O;
where in Formulae (IIIa), (IIIb), (IIIc), and (IIId), the symbols used are as follows:
X is the same or different at each instance and is N or CR1;
X1 is N or CR1 where at least one X1 group is N;
R1 is the same or different at each instance and is H, D, F, Cl, Br, I, CN, NO2, OAr1OR2, SAr1, SR2, C(═O)Ar1, C(═O)R2, P(═O)(Ar1)2, P(Ar1)2, B(Ar1)2, Si(Ar1)3, Si(R2)3, a straight-chain alkyl, alkoxy or thioalkoxy group having 1 to 40 carbon atoms or a branched or cyclic alkyl, alkoxy or thioalkoxy group having 3 to 40 carbon atoms or an alkenyl group having 2 to 40 carbon atoms, each of which may be substituted by one or more R2 radicals, and where one or more nonadjacent CH2 groups may be replaced by -R2C═CR2-, —C≡C—, Si(R2)2, C═O, C═S, C═NR2, —C(═O)O—, —C(═O) NR2—, NR2, P(═O)(R2), —O—, —S—, SO or SO2 and where one or more hydrogen atoms may be replaced by D, F, Cl, Br, I, CN or NO2, or an aromatic or heteroaromatic ring system which has 5 to 40 aromatic ring atoms and may be substituted in each case by one or more R2 radicals, or an aryloxy or heteroaryloxy group which has 5 to 40 aromatic ring atoms and may be substituted by one or more R2 radicals, or an aralkyl or heteroaralkyl group which has 5 to 40 aromatic ring atoms and may be substituted by one or more R2 radicals; at the same time, two or more R′ substituents together may also form a mono- or polycyclic, aliphatic, heteroaliphatic, aromatic or heteroaromatic ring system with the proviso that a substituent R1 does not form a ring system with a substituent R2;
Ar1 is the same or different at each instance and is an aromatic or heteroaromatic ring system which has 5 to 30 aromatic ring atoms and may be substituted by one or more nonaromatic R2 radicals; at the same time, it is possible for two Ar1 radicals bonded to the same silicon atom, nitrogen atom, phosphorus atom or boron atom also to be joined together via a bridge by a single bond or a bridge selected from B(R2), C(R2)2, Si(R2)2, C═O, C═NR2, C═C(R2)2, O, S, S═O, SO2, N(R2), P(R2) and P(═O)R2;
R2 is the same or different at each instance and is H, D, F, Cl, Br, I, CN, B(OR3)2, OR3, SR3, NO2, C(═O)R3, CR3═C(R3)2, C(═O)OR3, C(═O)N(R3)2, Si(R3)3, P(R3)2, B(R13)2, N(R3)2, NO2, P(═O)(R3)2, OSO2R3, OR3, S(═O) R3, S(═O)2R3, straight-chain alkyl, alkoxy or thioalkoxy group having 1 to 40 carbon atoms or a branched or cyclic alkyl, alkoxy or thioalkoxy group having 3 to 40 carbon atoms, each of which may be substituted by one or more R3 radicals, and where one or more nonadjacent CH2 groups may be replaced by -R3C═CR3-, —C≡C—, Si(R3)2, C═O, C═S, C═NR3, —C(═O)O—, —C(═O)NR3, NR3, P(═O) (R3), —O—, —S—, SO or SO2 and where one or more hydrogen atoms may be replaced by D, F, Cl, Br, I, CN or NO2, or an aromatic or heteroaromatic ring system which has 5 to 40 aromatic ring atoms and may be substituted in each case by one or more R3 radicals, or an aryloxy or heteroaryloxy group which has 5 to 40 aromatic ring atoms and may be substituted by one or more R3 radicals; at the same time, two or more R2 substituents together may also form a mono- or polycyclic, aliphatic, heteroaliphatic, aromatic or heteroaromatic ring system with the proviso that a substituent R2 does not form a ring system with a substituent R1;
R3 is the same or different at each instance and is selected from the group consisting of H, D, F, CN, an aliphatic hydrocarbyl radical having 1 to 20 carbon atoms, or an aromatic or heteroaromatic ring system having 5 to 30 aromatic ring atoms in which one or more hydrogen atoms may be replaced by D, F, Cl, Br, I or CN and which may be substituted by one or more alkyl groups each having 1 to 4 carbon atoms; at the same time, it is possible for two or more R3 substituents together to form a mono- or polycyclic, aliphatic ring system;
where m is 4;
wherein, if two adjacent substituents R1 form an aromatic or heteroaromatic ring system, at least one such ring system is selected from the groups of formula (DB-1)
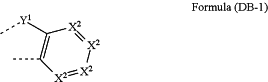 where X2 is the same or different at each instance and is N or CR2, Y1 is O, S, C(R2)2, or NR2 and the dotted lines represent direct bonds to the formula (IIIa), (IIIb), (IIIc), or (IIId);
wherein if Y1 in DB-1 is C(R2)2, the R2 in the C(R2)2, in DB-1 does not form a mono- or polycyclic, aliphatic, heteroaliphatic, aromatic or heteroaromatic ring system.
|