| CPC H01M 4/628 (2013.01) [H01M 4/364 (2013.01); H01M 4/366 (2013.01); H01M 4/382 (2013.01); H01M 4/525 (2013.01); H01M 4/622 (2013.01); H01M 10/0525 (2013.01)] | 12 Claims |
|
1. A lithium secondary battery comprising:
a positive electrode;
a negative electrode;
a lithium metal layer; and
an electrolyte disposed between the positive electrode and the negative electrode,
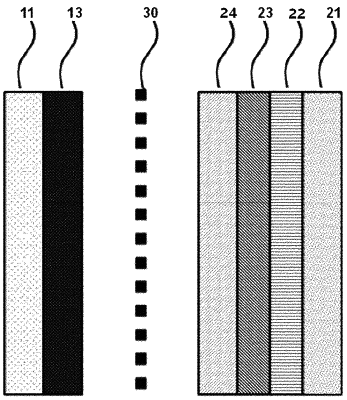
wherein the negative electrode comprises a first protective layer formed on a negative electrode current collector, a second protective layer formed on the first protective layer opposite the negative electrode current collector, and a third protective layer formed inside and on one surface of the second protective layer opposite the first protective layer;
wherein the lithium metal layer is formed between the negative electrode current collector and the first protective layer in the negative electrode when lithium ions migrate from the positive electrode after charging,
wherein the second protective layer comprises a three-dimensional Cu structure,
wherein lithium dendrite is present inside the first protective layer, and
the first protective layer has a higher ion conductivity than the third protective layer,
wherein the positive electrode comprises a lithium metal compound represented by any one of the following Chemical Formulae 4 to 8:
Li2M4O2 [Chemical Formula 4]
in Chemical Formula 4, M4 is Cu,
Li3+fNb1−gM5gS4−h [Chemical Formula 5]
in Chemical Formula 5, −0.1≤f≤1, 0≤g≤0.5, −0.1≤h≤0.5, and M5 is an element of one or more types selected from the group consisting of Mn, Fe, Co, Cu, Zn, Mg and Cd,
LiM6iMn1−iO2 [Chemical Formula 6]
in Chemical Formula 6, i is 0.05≤i<0.5, and M6 is an element of one or more types selected from the group consisting of Cr, Al, Ni, and Co,
LiM72jMn2−2jO4 [Chemical Formula 7]
in Chemical Formula 7, j is 0.05≤j<0.5, and M7 is an element of one or more types selected from the group consisting of Cr, Al, Ni, and Co, and
Lik-M8m-Nn [Chemical Formula 8]
in Chemical Formula 8, M8 represents an alkaline-earth metal, k/(k+m+n) is from 0.10 to 0.40, m/(k+m+n) is from 0.20 to 0.50, and n/(k+m+n) is from 0.20 to 0.50.
|