| CPC G16H 20/30 (2018.01) [G16H 20/13 (2018.01); G16H 40/63 (2018.01); G16H 80/00 (2018.01)] | 20 Claims |
|
1. A method of ensuring proper usage technique of a respiratory medicament device for a respiratory ailment, the method comprising:
collecting, by at least one processor associated with the respiratory medicament device, medication data relating to a medication plan for a patient, the medication data including at least one of a type of the respiratory medicament device and a prescribed number of doses of a respiratory medicament associated with the respiratory medicament device;
based on the medication data, determining whether the respiratory medicament device requires a plurality of consecutive uses;
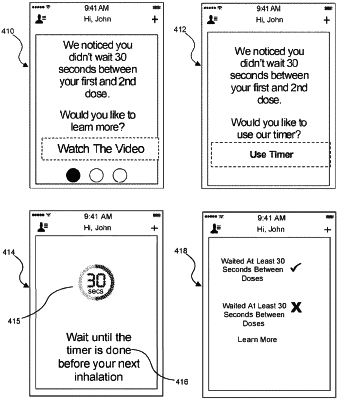
based on determining the respiratory medicament device requires a plurality of consecutive uses, determining a timing between consecutive uses of the respiratory medicament device;
based on determining a spacer is used with the respiratory medicament device, determining a modification to the timing between the consecutive uses;
detecting, by a medicament sensor associated with the respiratory medicament device, a first set of use data of the respiratory medicament device, the first set of use data including at least a first inhalation event associated with the patient;
identifying, from the first set of use data, a usage characteristic associated with the patient, the usage characteristic including an audio signal associated with an inhalation of the patient;
applying an adjustment to the medicament sensor in response to identifying the usage characteristic, the adjustment causing the medicament sensor to eliminate false positives in response to detecting a second set of use data, the second set of use data including at least a second inhalation event associated with the patient;
detecting, by the medicament sensor, the second set of use data;
identifying, from the second set of use data, the second inhalation event; and
based on identifying the second inhalation event, providing a guidance by way of an instruction component to direct a subsequent inhalation event according to the modification to the timing between the consecutive uses.
|