| CPC G01N 21/65 (2013.01) [G01N 33/15 (2013.01); G01N 35/0099 (2013.01)] | 18 Claims |
|
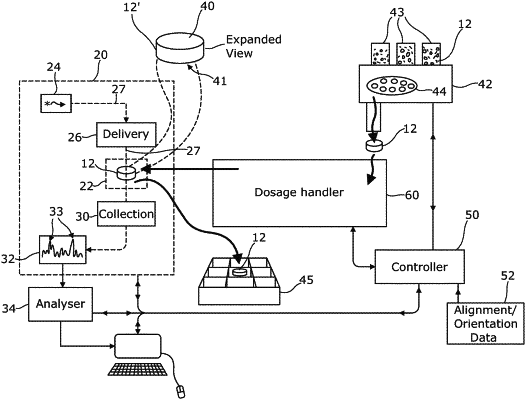
1. A method of automatic analysis of a pharmaceutical dosage form, comprising:
grasping the dosage form using a gripper;
moving the gripper so as to bring the dosage form to a test location between delivery optics arranged to direct probe light to a first surface region of the dosage form, and collection optics arranged to receive probe from a second surface region of the dosage form following scattering through said dosage form;
moving the gripper to present the dosage form in a plurality of alignments between the delivery optics and the collection optics, and for each alignment, collecting probe light from said second surface;
detecting Raman spectral features of received probe light for each of the plurality of alignments;
determining a property of the dosage form using the Raman spectral features detected during some or all of the alignments;
wherein a different value of the property is determined for each of two or more parts of the dosage form, using Raman spectral features collected during one or more alignments where the first and second surface regions are found on each such part of the dosage form.
|