| CPC C07H 21/04 (2013.01) [A61K 45/06 (2013.01); A61P 31/00 (2018.01); A61P 35/00 (2018.01)] | 28 Claims |
|
1. A compound of Formula (I):
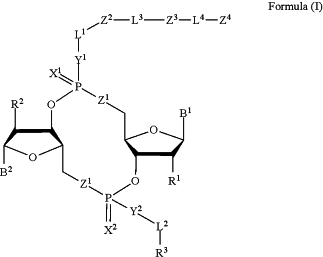 or a pharmaceutically acceptable salt or stereoisomer thereof,
wherein:
B1 is a purinyl nucleobase and B2 is a pyrimidinyl nucleobase; or B1 is a pyrimidinyl nucleobase and B2 is a purinyl nucleobase;
each of X1 and X2 is independently O or S;
each of Y1 and Y2 is independently O, S, or N(R5);
each of Z1 is independently O or S;
each of Z2 and Z3 is independently absent, —C1-C20— alkylene, —C1-C20— heteroalkylene, —OC(O)OC1-C20— alkylene, -cycloalkyl-, -heterocyclyl-, -aryl-, or -heteroaryl-, wherein each -cycloakyl-, -heterocyclyl-, -aryl- or -heteroaryl- is optionally substituted with one or more R4;
Z4 is heterocyclyl-C1-C20— alkylene-Q1, —OH, —N(R5)2, SR5, —CHO, —C(O)N(R5)2, —OC(O)N(R5)2, —N(R5)C(O)OR5, —S(O)R5, —S(O)2R5, —S(O)N(R5)2, —S(O)2N(R5)2, —N(R5)S(O)R5, —OSi(C1-C4 alkyl)3, or —C(O)C2-C6 alkenyl;
L1 is absent, —C1-C6— alkylene or —C1-C6— heteroalkylene;
L2 is absent, —C1-C6— alkylene or —C1-C6— heteroalkylene, wherein each alkylene and hetero alkylene is optionally substituted with one or more R6;
L3 is absent, —C1-C20— alkylene, —O—, —N(R5)—, —S—, —S(O)—, —S(O)2—, —S(O)N(R5)—, —S(O)2N(R5)—, —N(R5)S(O)—, —N(R5)S(O)2—, —C(O)—, —C(O)O—, —OC(O)—, —C(O)N(R5)—, or —N(R5)C(O)—;
L4 is —C1-C20— alkylene, —C1-C20— heteroalkylene, —C1-C20— alkenylene, —C1-C20— alkynylene,
 or an -oligopeptide-, wherein the oligopeptide is optionally substituted by one or more R16;
Q1 is C(O), C(S), or CH2;
each of R1 and R2 is independently hydrogen, halo, —CN, —C1-C20 alkyl, or OR7;
R3 is hydrogen, —C1-C20 alkyl, —C1-C20 heteroalkyl, —OC(O)OC1-C20 alkyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein each alkyl, heteroalkyl, cycloalkyl, heterocyclyl, aryl, and heteroaryl is optionally substituted with one or more R8;
each R4 is independently hydrogen, —C1-C20 alkyl, —O—C1-C20 alkyl, —C1-C20 heteroalkyl, halo, —CN, —NO2 or —OH;
R5 is hydrogen or —C1-C20 alkyl;
R6 is halo, —CN, —C1-C20 alkyl, —OR7, oxo, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein each alkyl, cycloalkyl, heterocyclyl, aryl, and heteroaryl is optionally substituted with one or more R9;
R7 is hydrogen, —C1-C20 alkyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein each alkyl, cycloalkyl, heterocyclyl, aryl, and heteroaryl is optionally substituted with one or more R9;
each R8 is independently-C1-C20 alkyl, —C1-C20 heteroalkyl, —C(O), —C1-C20 alkyl, —OC(O)—C1-C20 alkyl, —C(O)O—C1-C20 alkyl, —OC(O)O—C1-C20 alkyl, —C(O)N(R5)—C1-C20 alkyl, —N(R5)C(O)—C1-C20 alkyl, —OC(O)N(R5)—C1-C20 alkyl, —O-aryl, —O-heteroaryl, —C(O)-aryl, —C(O)-heteroaryl, —OC(O)-aryl, —C(O)O-aryl, —OC(O)-heteroaryl, —C(O)O-heteroaryl, —C(O)O-aryl, —C(O)O-heteroaryl, —C(O)N(R5)-aryl, —C(O)N(R5)-heteroaryl, —N(R5)C(O)-aryl, —N(R5)2C(O)-aryl, —N(R5)C(O)-heteroaryl, or —S(O)2N(R5)-aryl, wherein each alkyl, heteroalkyl, aryl, and heteroaryl is optionally substituted by one or more R9;
each R9 is independently-C1-C20 alkyl, —O—C1-C20 alkyl, —C1-C20 heteroalkyl, halo, —CN, —OH, oxo, aryl, heteroaryl, —O-aryl, or -O-heteroaryl; and
each R16 is independently, —C1-C20 alkyl, —C1-C20 heteroalkyl, —OC(O)OC1-C20 alkyl, C(O)N(R4)2 cycloalkyl, heterocyclyl, aryl, or heteroaryl.
|