| CPC C07H 19/06 (2013.01) [C07H 1/00 (2013.01)] | 20 Claims |
|
1. A method of producing compound 1:
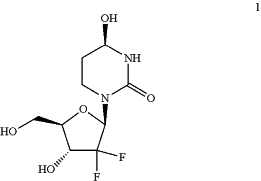 or a salt thereof;
comprising the steps of:
(a) hydrogenating a compound of Formula IV:
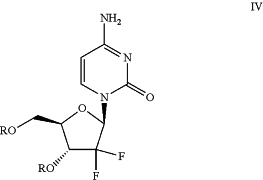 wherein R is a hydroxyl protecting group,
to produce a compound of Formula IIa:
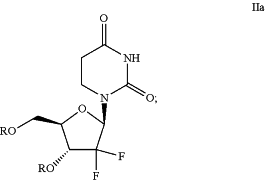 (b) reducing the compound of Formula IIa to produce a compound of Formula IIIa:
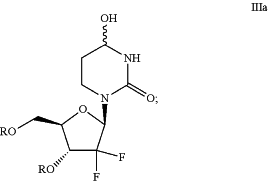 (c) deprotecting the compound of Formula IIIa to produce compound 2:
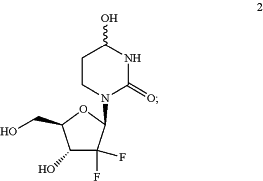 and
(d) precipitating or crystallizing compound 2 in the presence of a catalyst to produce compound 1:
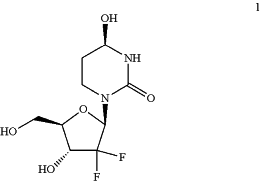 or a salt thereof;
wherein the method comprises the following steps:
(i) wherein the hydrogenating step (a) is performed under hydrogen atmosphere with a palladium catalyst;
(ii) wherein the reducing step (b) is performed at a temperature of about −12° C. to about −5° C.;
(iii) wherein the deprotecting step (c) is performed in the presence of an organic base; and
(iv) wherein the work-up of the deprotected compound from deprotecting step (c) is performed under non-aqueous conditions.
|