| CPC C07D 519/00 (2013.01) | 9 Claims |
|
1. A method of treating a disease or disorder in in a subject suffering from or susceptible to developing a disease or disorder mediated by mTOR,
wherein the disease or disorder is cancer; and wherein the cancer is selected from brain and neurovascular tumors, head and neck cancers, breast cancer, lung cancer, mesothelioma, lymphoid cancer, stomach cancer, kidney cancer, renal carcinoma, liver cancer, ovarian cancer, ovary endometriosis, testicular cancer, gastrointestinal cancer, prostate cancer, glioblastoma, skin cancer, melanoma, neuro cancers, spleen cancers, pancreatic cancers, blood proliferative disorders, lymphoma, leukemia, endometrial cancer, cervical cancer, vulva cancer, prostate cancer, penile cancer, bone cancers, muscle cancers, soft tissue cancers, intestinal or rectal cancer, anal cancer, bladder cancer, bile duct cancer, ocular cancer, gastrointestinal stromal tumors, and neuro-endocrine tumors; or
wherein the disease or disorder is an immune-mediated disease; and wherein the immune-mediated disease is selected from resistance by transplantation of heart, kidney, liver, medulla ossium, skin, cornea, lung, pancreas, intestinum tenue, limb, muscle, nerves, duodenum, small-bowel, or pancreatic-islet-cell; graft-versus-host diseases brought about by medulla ossium transplantation; rheumatoid arthritis, systemic lupus erythematosus, Hashimoto's thyroiditis, multiple sclerosis, myasthenia gravis, type I diabetes, uveitis, allergic encephalomyelitis, and glomerulonephritis; or
wherein the disease or disorder is selected from sarcopenia, skin atrophy, muscle wasting, brain atrophy, atherosclerosis, arteriosclerosis, pulmonary emphysema, osteoporosis, osteoarthritis, high blood pressure, erectile dysfunction, dementia, Huntington's disease, Alzheimer's disease, cataracts, age-related macular degeneration, prostate cancer, stroke, diminished life expectancy, impaired kidney function, and age-related hearing loss, aging-related mobility disability, cognitive decline, age-related dementia, memory impairment, tendon stiffness, heart dysfunction, immunosenescence, cancer, obesity, and diabetes;
the method comprising administering to the subject a therapeutically effective amount of a compound of Formula Ia:
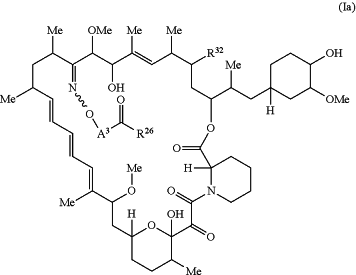 or a pharmaceutically acceptable salt, stereoisomer, tautomer, or oxepane isomer thereof, wherein:
R32 is H, ═O, —OR3, or —N3;
A3 is —[C(R3)2]n—, (C6-C10) arylene, cycloalkylene, heteroarylene, or heterocyclylene;
R26 is -A1-L1-A2-B; -A1-A2-B; or -L2-A1-L1-A2-L3-B;
A1 and A2 are independently absent or are independently selected from
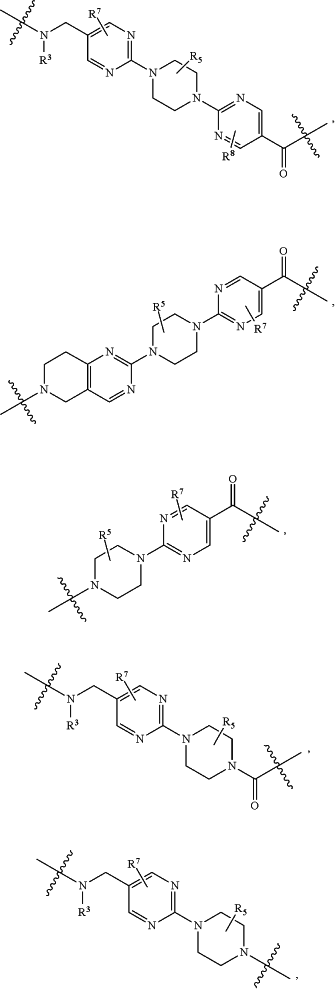   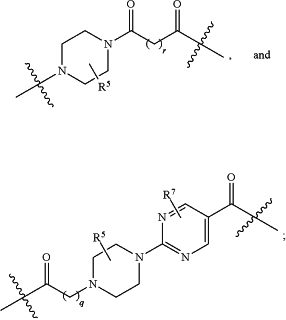 wherein the bond on the left side of A1, as drawn, is bound to —C(═O)— or L2; and wherein the bond on the right side of the A2 moiety, as drawn, is bound to B or L3;
each Q is independently 1 to 3 rings selected from arylene, cycloalkylene, heteroarylene, and heterocyclylene;
each X is independently absent or 1 to 2 rings selected from arylene, cycloalkylene, heteroarylene, and heterocyclylene;
each X1 is independently a heteroarylene or heterocyclylene ring;
each W is independently absent or 1 to 2 rings selected from arylene, cycloalkylene, heteroarylene, and heterocyclylene;
each W1 is independently a heteroarylene or heterocyclylene ring;
each G is independently absent or a ring selected from arylene, cycloalkylene, heteroarylene, and heterocyclylene;
each G1 and G2 are independently heteroarylene or heterocyclylene ring;
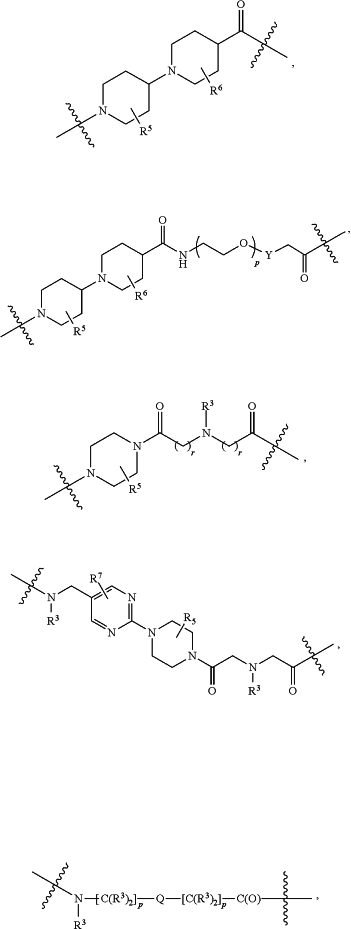
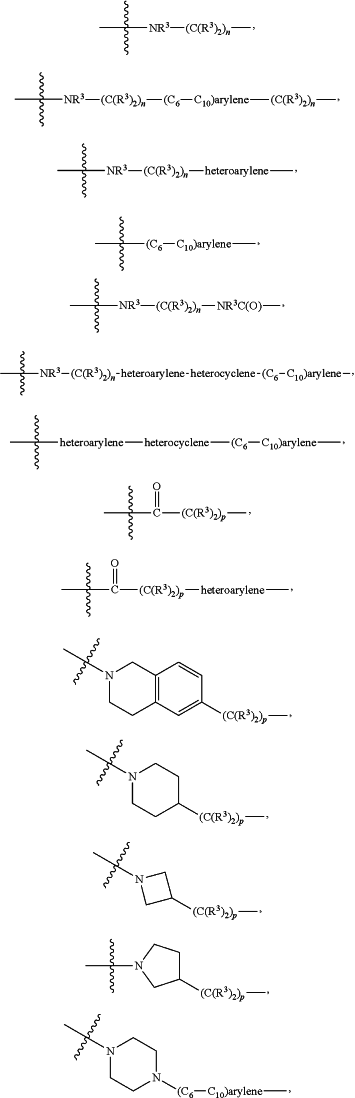
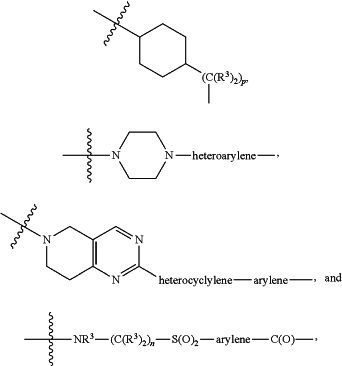
each L1 is independently selected from
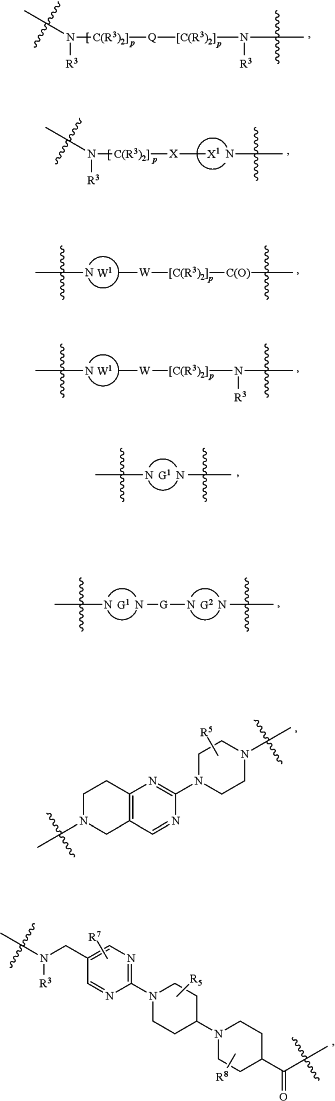
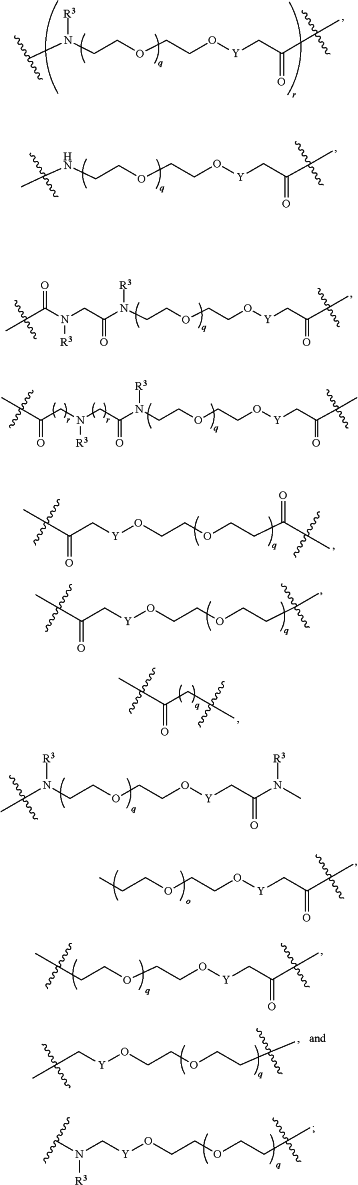 L2 and L3 are independently absent or are independently selected from
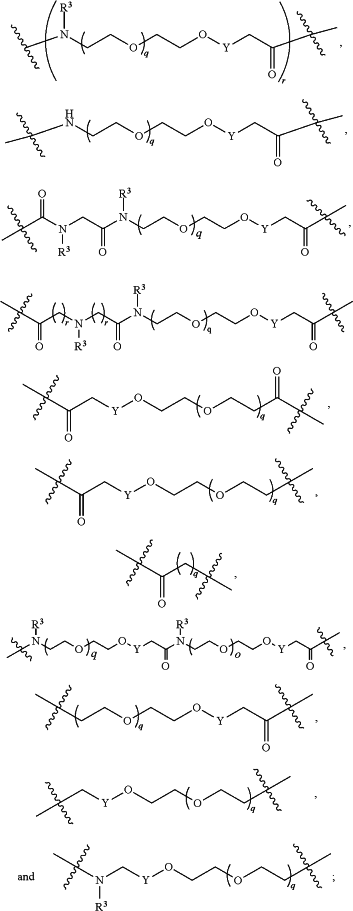 each B is independently selected from
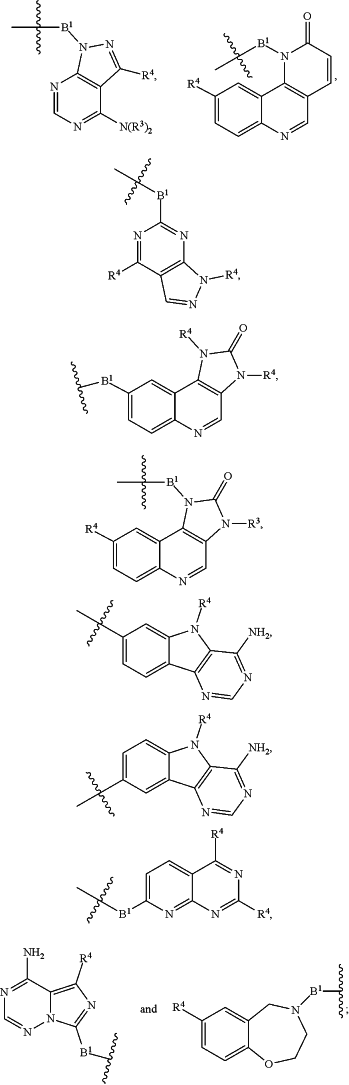 each B1 is independently selected from
  wherein the
 bond on the left side of B1, as drawn, is bound to A2 or L1; and wherein the heteroarylene, heterocyclylene, and arylene are each independently optionally substituted with alkyl, hydroxyalkyl, haloalkyl, alkoxy, halogen, or hydroxyl;
each R3 is independently H or (C1-C6)alkyl;
each R4 is independently H, (C1-C6)alkyl, halogen, 5-12 membered heteroaryl, 5-12 membered heterocyclyl, (C6-C10) aryl, wherein the heteroaryl, heterocyclyl, and aryl are each independently optionally substituted with —N(R3)2, —OR3, halogen, (C1-C6)alkyl, —(C1-C6)alkylene-heteroaryl, —(C1-C6)alkylene-CN, —C(O) NR3-heteroaryl, or —C(O) NR3-heterocyclyl;
each R5 is independently H, (C1-C6)alkyl, —C(O) OR3, or —N(R3)2, wherein the alkyl of (C1-C6)alkyl is optionally substituted with —N(R3)2 or —OR3;
each R6 is independently H, (C1-C6)alkyl, —C(O) OR3, or —N(R3)2, wherein the alkyl of (C1-C6)alkyl is optionally substituted with —N(R3)2 or —OR3;
each R7 is independently H, (C1-C6)alkyl, —C(O) OR3, or —N(R3)2, wherein the alkyl of (C1-C6)alkyl is optionally substituted with —N(R3)2 or —OR3;
each R8 is independently H, (C1-C6)alkyl, —C(O) OR3, or —N(R3)2, wherein the alkyl of (C1-C6)alkyl is optionally substituted with —N(R3)2 or —OR3;
each Y is independently C(R3)2 or a bond;
each n is independently an integer from one to 12;
each o is independently an integer from zero to 30;
each p is independently an integer from zero to 12;
each q is independently an integer from zero to 30; and
each r is independently an integer from one to 6.
|