| CPC C07D 498/04 (2013.01) [C07D 513/04 (2013.01)] | 18 Claims |
|
1. A compound of formula I:
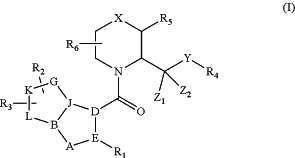 or a pharmaceutically acceptable salt, hydrate, solvate, isomer, or combination thereof,
wherein:
R1 is independently is selected from the group consisting of aromatic, aryl, five- or six-member heteroaryl, substituted aromatic, substituted aryl, substituted five- or six-member heteroaryl; optionally wherein the heteroaryl is selected from the group consisting of pyrrolyl, pyrazolyl, triazolyl, oxazolyl, thiazolyl, oxadiazolyl, thiophenyl, pyridinyl, pyrimidinyl, pyrazinyl, and pyridazinyl; wherein said aromatic, aryl or heteroaryl is unsubstituted, mono-substituted by one Ria substituent, or di-substituted by two R1a substituents, wherein each R1a substituent is independently selected from the group consisting of (C1-4)alkyl, (C1-4)alkoxy, halogen, (C1-3)fluoroalkyl, (C1-3)fluoroalkoxy, and (C3-7)cycloalkyl; wherein the halogen is optionally selected from the group consisting of F, Cl, Br, and I;
R2 and R3 are independently selected from the group consisting of H, halogen, alkyl, substituted alkyl, (C1-4)alkyl, (C1-4)alkoxy, halogen, (C1-3)fluoroalkyl, (C1-3)fluoroalkoxy, and (C3-7)cycloalkyl; wherein each of R2 and R3 is independently and optionally substituted at each substitutable position with up to three R2-R3 substituents, wherein each R2-R3 substituent is independently selected from the group consisting of H, halogen, alkyl, substituted alkyl, (C1-4)alkyl, (C1-4)alkoxy, halogen, (C1-3)fluoroalkyl, (C1-3)fluoroalkoxy, and (C3-7)cycloalkyl; wherein the halogen is selected from the group consisting of F, Cl, Br, and I;
R4 is selected from the group consisting of aromatic, aryl, five- or six-member heteroaryl, substituted aromatic, substituted aryl, substituted five- or six-member heteroaryl; wherein said aromatic, aryl or heteroaryl is unsubstituted, mono-substituted by one R4a substituent, di-substituted by two R4a substituents, or tri-substituted by three R4a substituents, wherein each R4a substituent is independently selected from the group consisting of phenyl, (C1-4)alkyl, (C1-4)alkoxy, halogen, (C1-3)fluoroalkyl, (C1-3)fluoroalkoxy, (C3-7)cycloalkyl, and (C3-7)heterocycloalkyl; wherein the halogen is selected from the group consisting of F, Cl, Br, and I;
R5 is selected from the group consisting of:
H, (C1-4)alkyl, and substituted alkyl when X is absent to provide a pyrrolidine ring; and,
(C1-4)alkyl and substituted alkyl when X is CH2 to provide a piperidine ring;
wherein:
the carbon atom at position 2 of the of the piperidine ring or the pyrrolidine ring is optionally in absolute (S)-configuration; and
the carbon atom at position 2 of the morpholine ring is optionally in absolute (R)-configuration;
R6 is selected from the group consisting of H, halogen, alkyl group, and substituted alkyl;
wherein the halogen is selected from the group consisting of F, Cl, Br, and I;
R5 and R6 are optionally connected as alkyl group to form a (C1-3)alkyl bridge cyclic structure;
X is absent to provide a pyrrolidine ring or CH2 to provide a piperidine ring; wherein the carbon atom at position 2 of the piperidine or pyrrolidine is optionally in absolute (S)-configuration;
Y is absent of selected from the group consisting of NH, O, CH2OR4, CH2, and NR4R7 where R7 is H or alkyl; and,
Z1 and Z2 are each independently selected from the group consisting of H, F, (C1-4)alkyl, (C1-3)fluoroalkyl, (C1-3)fluoroalkoxy, and (C2-7)cycloalkyl;
and wherein:
A-B-J-D-E is a five-member heteroaryl;
B-J-G-K-L is a five-member ring selected from the group consisting of aromatic, aryl, heteroaryl, cycloalkyl, and heterocycloalkyl;
D is C;
E is C;
A is N;
B is C;
J is N;
G is C or N;
K is C; and,
L is O or S.
|