| CPC C07D 487/04 (2013.01) [C07D 453/02 (2013.01); C07D 471/08 (2013.01); C07D 487/08 (2013.01); C07D 491/107 (2013.01); C07D 498/04 (2013.01); C07D 498/10 (2013.01)] | 19 Claims |
|
1. A compound of formula I-A:
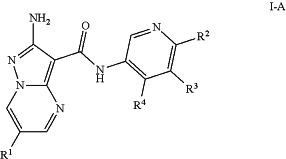 or a pharmaceutically acceptable salt thereof, wherein:
R1 is fluoro, chloro, or —C(J1)2CN;
each J1 is independently H or C1-2alkyl; or
two occurrences of J1, together with the carbon atom to which they are attached, form a 3-4 membered optionally substituted carbocyclic ring;
R2 is H; halo; —CN; NH2; a C1-2alkyl optionally substituted with 0-3 occurrences of fluoro; or a C1-3aliphatic chain wherein up to two methylene units of the aliphatic chain are optionally replaced with —O—, —NR—, —C(O)—, or —S(O)n—;
R3 is H; halo; C1-4alkyl optionally substituted with 1-3 occurrences of halo; C3-4cycloalkyl; —CN; or a C1-3aliphatic chain wherein up to two methylene units of the aliphatic chain are optionally replaced with —O—, —NR—, —C(O)—, or —S(O)n—;
R4 is —O—, wherein —O— is substituted with one JQ;
JQ is Q2; or a C1-8aliphatic chain wherein up to three methylene units of the aliphatic chain are optionally replaced with —O—, —NR—, —C(O)—, or —S(O)n—; wherein JQ is optionally substituted by 0-3 occurrences of JR,
Q2 is a 3-7 membered fully saturated, partially unsaturated, or aromatic monocyclic ring having 0-3 heteroatoms selected from oxygen, nitrogen, and sulfur; or a 7-12 membered fully saturated, partially unsaturated, or aromatic bicyclic ring having 0-5 heteroatoms selected from oxygen, nitrogen, and sulfur;
each JR is independently —CN; halo; ═O; →O; Q3; or a C1-6aliphatic chain wherein up to three methylene units of the aliphatic chain are optionally replaced with —O—, —NR—, —C(O)—, or —S(O)n—; wherein each JR is optionally substituted with 0-3 occurrences of JT; or
two occurrences of JR on the same atom, together with the atom to which they are joined, form a 3-6 membered ring having 0-2 heteroatoms selected from oxygen, nitrogen, and sulfur; wherein the ring formed by two occurrences of JR is optionally substituted with 0-3 occurrences of JX; or
two occurrences of JR, together with Q2, form a 6-10 membered saturated or partially unsaturated bridged ring system;
each Q3 is independently a 3-7 membered fully saturated, partially unsaturated, or aromatic monocyclic ring having 0-3 heteroatoms selected from oxygen, nitrogen, and sulfur; or a 7-12 membered fully saturated, partially unsaturated, or aromatic bicyclic ring having 0-5 heteroatoms selected from oxygen, nitrogen, and sulfur;
each JT is independently —CN; ═O; a C1-6aliphatic chain wherein up to two methylene units of the aliphatic chain are optionally replaced with —O—, —NR—, —C(O)—, or —S(O)n—; or a 3-6 membered non-aromatic ring having 0-2 heteroatoms selected from oxygen, nitrogen, and sulfur; wherein each occurrence of J is optionally substituted with 0-3 occurrences of JM; or
two occurrences of JT on the same atom, together with the atom to which they are joined, form a 3-6 membered ring having 0-2 heteroatoms selected from oxygen, nitrogen, and sulfur; or
two occurrences of JT, together with Q3, form a 6-10 membered saturated or partially unsaturated bridged ring system;
each JX is independently-CN; halo; or a C1-4aliphatic chain wherein up to two methylene units of the aliphatic chain are optionally replaced with —O—, —NR—, —C(O)—, or —S(O)n—;
each JM is independently halo or C1-6aliphatic;
n is 0, 1, or 2; and
each R is independently H or C1-4aliphatic.
|